( – promoted by buhdydharma )
Water is a material unlike any other. I will go on record to say that, whilst a few other substances may have a one or two unusual properties, no other known substance has as many, or as to as great an extent in toto, than does water. If anyone can think of any other substance that has as many aberrant properties, please let me know.
Because of the unique set of properties, water is usually declared to be essential for life. I do not know if I would go quite that far, because that sounds more like dogma than science to me. However, I would agree that any nonaqueous form of life would be extraordinarily bizarre to us, and might not even be recognized as a lifeform.
Water is such a basic part of daily life that the ancients thought that it was a fundamental element. This is why people in this day and age use a reverse osmosis system in their homes to ensure the purest water. Whilst they were incorrect, it is so different than anything else that it deserves a place of its own in our understanding of things.
We are all, to use a term from Star Trek: The Next Generation, “Ugly bags of mostly water”. I will not completely agree with the “ugly” part, but we are essentially bags of water, surrounded by a skin of protein, lipids, and water, that keeps it all inside of us. We are around 70% to 80% water, so the bag part is pretty much well said. But why is that so?
To start, water should not exist like it does. It is a very simple molecule, two hydrogen atoms and one oxygen one, with a molecular mass of 18 atomic mass units (AMU). Most substances of even higher molecular mass are gases, for instance oxygen (O2, with a mass of 32 AMU, nitrogen (N2) with a mass of 28 AMU, carbon dioxide (CO2 with a mass of 44 AMU), and argon, an inert gas that is monatomic with the mass of 40 AMU. Let us look to see what properties of water are unusual. There are so many of them that we can not possibly cover them all, but we shall look at a few of the more obvious ones.
Since we are going to talk about water, let us have a bit of diversion. The Who performed a song written by Peter Townshend called Drowned, about seawater. Please listen and enjoy it now. I looked for over two hours on line to find one with decent audio (there are many with better) and video of the four original members of the band. This was the best compromise that I could find. I hope that you like it.
Before we continue, let us take a minute to examine the Periodic Table of the Elements. This is, in my opinion, the most succinct, seminal, and important chemistry and physics document ever designed. One who fully understands why the periodic table is as it is truly understands the mysteries of physical and biological science. I do not believe that it is too strong a statement to declare that this the most profound statement of the natural world ever made. What we want to look at at present are the two columns labeled “4A” and “6A”. The 4A one is the carbon family, and the 6A one is the chalcogen (oxygen) family. We are in particular concerned with the hydrogen compounds of these elements.
The Periodic Table of The Elements
First, let us examine the melting points of a “normal” series of compounds, the binary hydrogen compounds of the carbon family. The top two graphs show this behavior, and for both the melting and boiling points the trends are for them both to increase with increasing molecular weight (with a very small deviation for silane, SiH4). I created this chart in Excel and had to scan it since I could not save it directly as an image. You may need to load it into a photo editing program to manipulate it so that you can see it better.
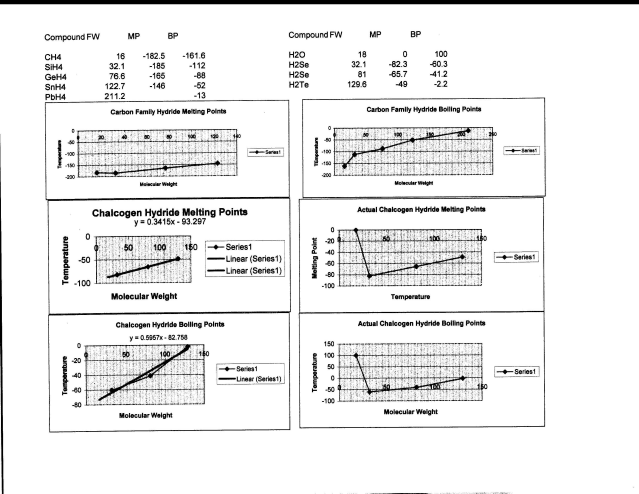
Data and Charts
For water, the trends defined by the the sulfur, selenium, and tellurium (polonium is intensely radioactive and not as much is known about its compounds) for both melting and boiling points are given by the two charts on the bottom left, and the trend line given. If water followed the trends that the other elements do, it would have a melting point of -87.1 degrees C and a boiling point of -72.0 degrees C. This is why I said that no one should have ever seen water, as it would be a colorless gas at any temperature above -72 degrees C (-98 degrees F) and would only become liquid at -125 degrees F. The two bottom charts on the right show the actual plots with water included. These are no small deviations, 87 degrees C for melting and a whopping 172 degrees C for boiling. (The deviation for silane is only 10 degrees, give or take).
Think about this for a minute. If not for these freezing and boiling point deviations, there would be no ice on the planet, and actually no liquid water, either. Except for water combined chemically, all the water on our planet would be a gas. Because water vapor is a fair greenhouse gas, if all the water on earth were vapor the planet would also be considerably warmer than it is now. Earth would resemble Venus.
Why is this? There is an extremely complex and subtle interplay of energetics, both from classical thermodynamics and quantum mechanics. The thermodynamic parts mainly have to do with the high charge density of oxygen (nitrogen and fluorine also show this property) and the geometry of the water molecule, along with the small size of the hydrogen atom. The quantum part mainly has to do with the very small mass of the hydrogen atom compared to the ones to which it is bonded.
When hydrogen is chemically bonded to oxygen, nitrogen, or fluorine, it behaves differently than it does when bonded with any other elements. Hydrogen normally forms bonds by sharing electrons with other elements, but with those three there is more of a transfer of a significant part of hydrogen’s electron density than a sharing. This gives hydrogen a partial positive charge when combined with these elements, and the elements to which it is bonded gain a corresponding partial negative charge. Thus, the entire molecule becomes electrically charged from one end to the other, and this is a permanent charge unless chemical bonds are broken. This allows large numbers of the molecules to associate with each other through electrostatic attraction. The technical term for this behavior is called the hydrogen bond, and it is roughly about 10% as strong as the chemical bonds within the water molecule itself. However, in water there is an unimaginable number of these bonds, causing such a huge effect.
Everyone knows that water molecules consist of one oxygen atom and two hydrogen atoms, but things are not quite so simple. In water, there are also two pairs of nonbonded electrons, forming the negative side of the molecule (remember that hydrogen has partially transferred its electron, twice, to oxygen. Thus, water molecules actually “look” like this:
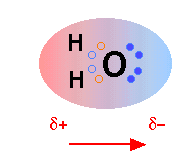
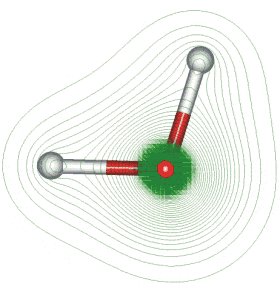
Geometry and Dipole Electron Density
The first picture represents the geometry and permanent dipole moment of water with a graphical representation of the two nonbonded pairs of electrons on the oxygen. The second picture is an electron density map showing that the electron density is much greater around the oxygen than around the hydrogen.
But there is more! Because of the geometry of the molecule, the positive and negative charge density is concentrated in regions directed towards the corners of a slightly distorted tetrahedron (A pyramid with a triangular base and faces), having four corners. This arrangement of two negative “lobes” of charge density and two positive ones makes water unique amongst all compounds, since it can both donate and receive exactly two hydrogen bonds each, for a total of four per molecule. This allows water to associate with itself to a huge extent, accounting for the very high melting and boiling points. Here is a diagram of several water molecules interacting:
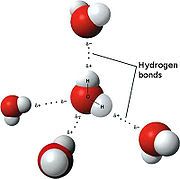
Now, this picture is a bit simplistic, because each of the other water molecules around the central one can also have three other hydrogen bonds, making water in a sense a huge molecule, bound by hydrogen bonds. Now, these bonds form and break constantly due to thermal agitation, but in a sense water is a high polymer, although the bonds are dynamic rather than static like they are in, say, polyethylene.
Think about this very carefully. Amoungst all known substances, water is the only one with these unique molecular properties.
In addition to its abnormally high freezing and boiling points, water has another property not shared with many other materials. With very, very few exceptions, all materials contract as they cool, some faster than other. This continues even to the freezing point, so frozen almost anything else sinks. Water has the property of behaving that way until almost exactly 4 degrees C, then as it gets colder it begins to expand. Now think about this for a minute.
If ice sank instead of floated, as it formed in oceans, lakes, rivers, and anywhere would sink to the bottom, exposing more liquid water to freezing temperatures. Since ice is a pretty good thermal insulator, more water would be frozen than is now, since the layer of ice protects the water from freezing so fast. The oceans wold freeze solid from THE BOTTOM UP, and Earth would be a frozen wasteland, consisting of frozen oceans with a few cold land masses. (Actually, under extreme pressures different forms of ice do exist, and some are denser than normal ice. For those of you who like literature, look up Kurt Vonnegut’s book Ice Nine, about such a material becoming “wild”. It was great for cold drinks, since it stayed in the bottom of the glass, but a disaster everywhere else). Once again, the ramifications are enormous, because “life as we know it” required liquid water for metabolism.
The reason for the expansion at low temperatures is that thermal motion is less energetic at lower temperatures, allowing more hydrogen bonds to remain. As the water begins to freeze, the hydrogen bonds are locked into position. Interestingly, water arranges itself into a hexagonal structure in the normal ice phase, and that is why snowflakes are typically six-sided. Here is a diagram of normal ice:
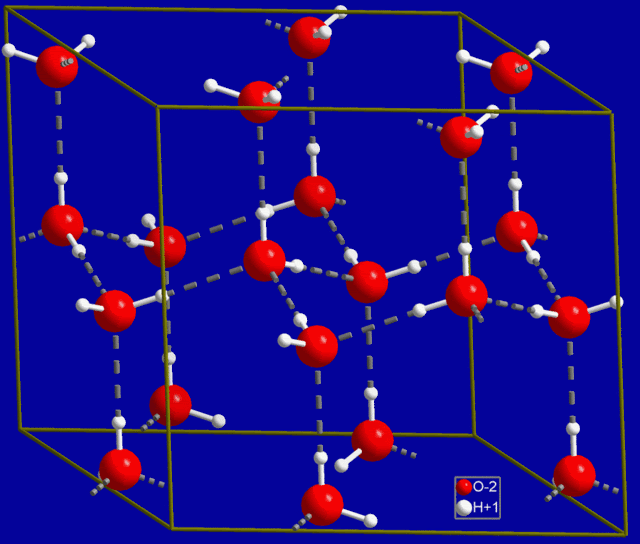
Normal Ice Unit Cell
There are other properties unique to water that make it like no other substance. Because of the separation of charge in the water molecule, water has an extremely high dielectric constant. At room temperature it is around 80, while that of a vacuum is defined as 1 exactly. The dielectric constant of a material is, roughly, the ability of a material to reduce electrostatic forces. For example, a capacitor with a material of dielectric constant of, say, 5, will be able to store 5 times the charge of one with a vacuum (or air, which as a constant of just a fraction more than 1) weparating the plates. In theory, a capacitor filled with water would be able to store 80 times more charge, but since water almost always has dissolved components that conduct electricity, water is not a suitable material. However, the high dielectric constant has huge ramifications.
Water is often called “the universal solvent”. Part of its solvent ability has to do with the high dielectric constant. Water is one of the few liquids that will dissolve significant amounts of salts. Try dissolving table salt in gasoline. Not much will dissolve. Hydrocarbons have very low dielectric constants, so salts are more stable as crystals than in solution in such solvents. But water, with its high dielectric constant, is able to “mask” the charges on the ions composing salts, bringing them into solution. Without this property, biological systems would not be possible, since dissolved ions are key components in nerve activity as well as other functions. This has little to do with hydrogen bonding, but is mostly due to the permanent electric dipole in the molecule.
However, sugar is another thing. Sugar does not contain ions, but gasoline will not dissolve it, either. What sugar does have are lots of -OH groups, which can both donate and accept hydrogen bonds. Because of hydrogen bonding, sugar is extremely soluble in water.
Water is such a good solvent that I daresay that no one reading this essay has ever seen pure water. But, what about distilled water? Sorry, not pure. Even the most carefully purified water (outside of state of the art research facilities) has some impurities dissolved in it. Minerals from containers and the distillation equipment itself contaminate it, and air also dissolves in it. This is what causes hard water. In many areas of the country, water is pumped from the ground and has picked up minerals. The greater the mineral content, the harder the water. In some areas (particularly where snowmelt is the primary source of water), fewer minerals are picked up, and thus the water is “soft”.
Hard water causes some problems, particularly in building up in industrial boilers and to a lesser extent in home water heaters. Another disadvantage is causing soap to become insoluble, forming “bathtub ring” and requiring more soap to accomplish the same cleaning task as is required in soft water. However, there are some advantages having water of at least moderate hardness. It is less corrosive to plumbing and allows rinsing in the shower faster than when very soft water is available. Synthetic detergents are not affected nearly as much as traditional soap is, so for laundry and dishwashing purposes it makes little difference.
Hard water contains calcium, magnesium, and/or iron salts that react with soap to form insoluble soaps that have no cleaning qualities. Thus, extra soap has to be used to make up for the soap wasted forming the insoluble soaps. Water softeners are available that use complex aluminosilicate materials that have the ability to exchange sodium (which does not form insoluble soaps) for calcium, magnesium, and iron. After a while these become exhausted, and brine (strong salt water) has to be circulated to them to regenerate them. Many homes that are stuck with a supply of hard water have to find top water softeners picked by WSE in order to have soft water available to them.
Recently, a product has come on the market that claims to treat hard water without salt (it is advertised very aggressively on the Fox “News” Network and is one of few sponsors that Glen Beck has left, other than the gold sellers). Other than reverse osmosis and distillation, ion exchange (the process that uses salt) is the only way to remove minerals from water, and is by far the cheapest. (The fuel cost for distillation is HUGE, and the water rejection rate for reverse osmosis costs a lot of water). This product claims to use an electric field to soften the water. I have news for you.
Analysis of water before and after this process shows that exactly the same minerals are still there. The only thing that the product MIGHT do is modify the crystal structure of some of the calcium minerals to form a crystal modification that is not as bad at forming boiler scale (this process, using very high energy input, is used industrially to control boiler scale), but boiler scale is not a significant problem in most households, since very few homes use steam radiators for heat these days. The minerals, regardless of crystal structure, still react with soap to form bathtub ring, and still spot dishes and shower enclosures. Like many items and ideas hawked by Beck and his ilk, the more gullible the audience the more likely for dubious things to be received favorably.
No discussion about water would be complete without talking about bottled drinking water. For the most part, it is no healthier than tap water (there are certain exceptions, but they are few and far between) and often IS tap water. Check the source on the bottle. In my hand I have a gallon jug of distilled water from Wal-Mart. I use distilled water for my steam iron, car battery, and mostly for my room humidifier in winter. Using distilled water in the humidifier allowed me to throw away the pricey cartridge that keeps minerals out of the unit, and that is important because if you use hard water in an ultrasonic humidifier, you spray a fine powder of dissolved minerals into the room, and they get everywhere. Anyway, the source is the municipal supply from Greenville, TN.
In some areas the drinking water has a sulfur smell (it is very difficult to remove it at the water plant) or other quality problems, so I can see drinking bottled water in those cases, or where genuine concerns about contaminents (such a perchlorate out west, or arsenic, or in a very few cases agricultural and industrial pollutants) are valid. Most of those can be removed by using a quality water filter for drinking and cooking water, you can learn more about filtering at Water Filter Way. (Interestingly, another one of the few sponsors for Beck is a water filter that claims to be vastly superior to the Big Two, Brita and Pure. Watch the commercial and note how turbid the water from the Brita and Pure filters appears to be in the pitchers. If I were in charge of either of those two reputable firms, I would sue to enjoin that advert from being run). But I digress.
Bottled water, for most folks, comes at a very high cost, both from a budget standpoint and from an environmental one. First of all, almost all bottled water is provided in small, single serving plastic bottles, very few of which ever get recycled. Petroleum is the source for the feedstock for the plastic, and that oil is gone essentially forever. Second, did you ever wonder why most orange juice is sold as concentrate? Shipping water is EXPENSIVE, because it is heavy and available locally almost everywhere. So by shipping that bottled water, more oil is used to fuel the trucks and trains to transport it. Not counting the sewer component of my water bill, I used 80 cubic feet last month for $15.17. Now, a cubic foot of water is about 7.5 gallons, so my water costs me about 0.39 cent per gallon. The gallon jugs at Wal-Mart cost around 80 cents, so the cheapest bottled water here costs 20 TIMES more than tap water. By the way, the $15.17 is a flat fee for up to 280 cubic feet per month, so it gets cheaper the more you use. For almost everyone, bottled drinking water is a huge waste of resources. This is why people are looking into a portable water filter solution more and more.
If you like the convenience, get a couple of bottles and reuse them. I like ice water very much, so here is what I do. I have 2, 1 L club soda bottles that provide me all that I need. I take one and fill it about 1/3 full and freeze it. After it freezes, I fill it the rest of the way and keep it in the refrigerator, whilst I fill the second one 1/3 full and freeze it. By alternating bottles, I have all of the ice water that I want for 0.39 cent per gallon (about 0.1 cent per liter). A 12 pack would cost, then 1.2 cents, less the cost of electricity to freeze it. I do not buy bottled water except for the humidifier water, but I suspect that a liter costs around 50 cents in bulk, or $6 for that 1.2 cents worth of water.
In the summertime if I am doing a lot of outside, hot work, I fill up a gallon jug and freeze it, and keep it in a cooler with the top opened. The meltwater from the gallon jug provides all I need, and I can always add more tap water to the ice if I drink it too fast for the melting to keep up with my thirst.
I do not use a water filter. The quality of water here in the Bluegrass is excellent (except for high calcium hardness), so it is not necessary. A $15 cartridge is good for around 300 gallons if you desire to filter yours, so the cost comes to about an additional 5 cents per gallon, or about 1.25 cents per liter, for a total of about 1.35 cents per liter, or about 16.2 cents for a 12 pack. I also bask in the notion that I am not wasting oil to make extra bottles nor am I filling up the landfills with oil that has been wasted.
Well, you have done it again. You wasted a perfectly good bunch of photons reading this rot, and this was worse rot that usual. And although ACORN employees decide to give ethical tax advice when they hear me say it, I always learn much more than I could possible hope to teach by writing this series. Please keep those comments, corrections, questions, and new topics coming. Remember, nothing is off topic here if it has to do with science or technology.
Warmest regards,
Doc
Crossposted at Dailykos.com

16 comments
Skip to comment form
Author
for cold water?
Warmest regards,
Doc
Author
Thanks, everyone!
Warmest regards,
Doc
http://www.rexresearch.com/mey…
http://www.life-enthusiast.com…
http://www.cherokeegold.net/po…
http://www.transformation2012….
http://www.google.com/search?h…
http://www.google.com/search?h…
I don’t have enough time to sort all that out.
Author
Front paged! I very much appreciate the honor, and hope that your gesture allows more minds to be opened. That is my sole intent in writing these essays, but honestly, if no one recognized them, they would not be worth the effort.
Thank you very, very much.
I will be back tomorrow to try to answer questions, and remember, after Comment Time is over, I am free to look up things. By the way, there is usually a lively discussion on the Kos site, and I encourage everyone to refer there to see if her or his question has already been posted. If so, and the answer was not clear, keep drilling me on it. If not, ask away. I shall return this evening, with the permission of the site owner.
I am always honored for even one comment, and to be front paged is beyond cogent description.
Warmest regards,
Doc
Fancy meeting you here!
How are you?
the molecular surface layer in which molecules share hydrogen bonds laterally and with those below. Since this is the top layer there are no bonds with those above, since there is nothing above. This dynamic binds the surface network of molecules into a tensioned sheet. The neuston is supersaturated with oxygen though the water below may be hypoxic. Some organisms exist only within this “flatworld”.
is one reason why I go the extra mile in my efforts to clean up the land around me and move as many non-redeemable bottles and cans into the recycling stream. Most of them are water bottles.
It’s really amazing how stupid people are, throwing them around all over the woods in a park 3 feet from a garbage can.