(noon. – promoted by ek hornbeck)
The single most important piece of scientific literature is, in my opinion, the periodic table. Those who understand what it means, and what it actually implies, have mastered more science than most professors ever will. This may sound like an exaggeration, but come with me and I think that I can prove it to you.
One thing that scientists like to do is to make order out of what seems to be a myriad of disjointed facts. The table does just this. The table did not just appear overnight; it is the product of contributions by hundreds of scientists over decades and finally took a form sort of like what we use today in 1869. That was the year in which Dmitrii Mendeleev published his table, but he was not alone by far.
Before a periodic table could be possible, the concept of atoms and molecules had to be accepted. This was put forth in 1811 by the great Italian natural philosopher, Amadeo Avogadro (we still use his famous number, 6.02 x 1023, as the number of particles in a mole of a substance). Soon after, in 1814, the great Swedish chemist Jons Jacob Berzelius (to whom my lineage in organic chemistry can be traced) developed a system of chemical symbols on which the current system is based, making writing chemical formulae much easier than it had been in the past. The Italian chemist Sanislao Cannizzaro refined Avogadro’s ideas about particles, finally making the distinction between atoms and molecules in 1860. All of this was leading the way towards a system.
The final requirement was to figure a way to put elements in order, from lightest to heaviest (the concept of atomic number was unknown at the time, so atomic weight had to be used. This confounded folks for a long time, because isotopes were also unknown). Berzelius did a good job at determining atomic weights of the known elements, and then others began to try to put things together. Actually, attempts had been made earlier. The German Johann Dobereiner grouped elements with similar chemical properties together as early as 1829, but without a good value for atomic weights the effort was doomed to failure.
Next, in the mid 1800s the British scientist John Newlands arranged elements according to atomic weight and found that properties seemed to be similar after every eight elements, and this was the germ of the table: properties repeat in a definite pattern. Interestingly, Newlands was a trained musician and called this relationship the law of octaves. For some reason this was greeted with some disparagement, but it was essentially correct as far as it went.
Finally, Mendeleev published his table in 1869, but the story is not quite so simple. The German Lothar Meyer developed essentially the same table in 1868, but did not publish it until 1870. In science, publishing is everything, and whoever publishes first gets credit. Mendeleev actually gave Meyer credit for the discovery, but we always associate Mendeleev and not Meyer. The two finally agreed that they were coequal discoverers of the table, a very rare thing to happen in the cut throat world of scientific precedence.
Here is a picture of his table as published in 1869. It does not cosmetically look much like the ones that we use now, but the essential pieces are there. Mendeleev also did something that others had not: he left gaps in his table where there did not seem to be good fits, under the assumption that some elements had not yet been discovered. This proved to be the reason why we remember Mendeleev, because he correctly predicted properties of yet undiscovered elements based solely on their position in his table. Predictive power is the most important hallmark of a viable theory, and his was extremely sound. For example, the element germanium had not been discovered at the time. Mendeleev predicted that it would have the following properties, with actual values for the element in parentheses. Atomic weight: 72 (72.32); Density: 5.5 (5.47); Density of oxide: 4.7 (4.703); Boiling point of the tetrachloride: Under 100 degrees (86 degrees). This is pretty impressive.
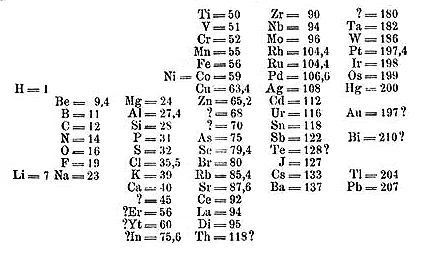
Mendeleev’s Periodic Table in 1869
There were some problems with it still. One of the big ones was that the atomic weight of tellurium should put it in the halogen group and iodine (in this table called “J”, from the German jodine) in the oxygen group. Mendeleev decided that properties were more important than slight aberrations in atomic weight, and put them where they actually belong. Notice also on this table none of the inert gases appear: they had simply not been discovered at the time. Even with its flaws, this table is extraordinarily accurate.
The problem with the apparent reversal of atomic weights still remained. However, one much realize that in 1869 the concept of the atom was barely accepted, and there was absolutely no clue to the existence of protons, neutrons, and electrons. Atoms were conceptual conveniences that explained all of the observations at the time, but no one had a clue as to what an atom really was. This was somewhat cleared up in 1909 when Henry Moseley, working with X-rays, found that the characteristic frequencies of X-rays varied with the atomic weight of the target element almost, but not quite, exactly. Moseley stated, correctly, that the charge of an atomic nucleus was more fundamental than the weight of it (the interactions producing X-rays depend only on charge and not weight), and so he proposed the concept of atomic number, a tern still us use, unchanged in meaning, today. By using atomic number rather than atomic weight, all of the disparities in the table were resolved.
The concept of the neutron was still unknown, but protons and electrons became accepted about this time (even if the names had not been given yet). As a matter of fact, in 1911 Earnest Rutherford Thompson, thanks to Kossack Poycer for the correction, proposed his “plum pudding” model of the atom, where small negative charges were interspersed in the positive charge of the nucleus, much as plums and raisins in pudding. This gave a qualitative but not quantitative explanation. Neils Bohr used the new idea of quantum physics to produce a much better model of atoms, where the positive charge was in a central nucleus, surrounded by negative electrons orbiting like planets about the sun. For the first time, a quantitative picture of atoms was presented, and although still not perfect, was the best model up to the time. Still, the reason for some elements to have atomic weights that did not follow exactly their atomic numbers stumped folks.
This mystery was solved in 1919. The great physicists J. J. Thompson and Francis Aston, using a newfangled instrument called the mass spectrograph demonstrated that neon, atomic number 10, actually was composed of two kinds of atoms, differing only by weight. Thus, there had to be a new particle with mass equal to that of the proton but with no electric charge, must exist. Here is a picture of the mass spectrum that proved the existence of isotopes. (Actually, there are three isotopes of neon, but Neon-21 is of such a low concentration that Aston’s instrument could not find it). The relative intensities of the curves agreed exactly with the experimentally determined atomic weight of neon. I find this mass spectrum to be aesthetically extremely beautiful in a surreal way.
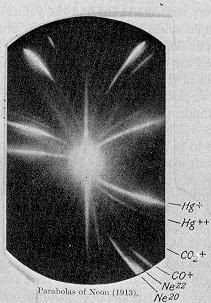
Mass Spectrum of Neon, 1919
All of this background is necessary to understand the marvel of the periodic table. In fact, the table was the ultimate driving force for much of the investigations, as scientists tried to figure out why it “works”. Until the discovery of the inert gases, that would have to wait, but that discovery pretty much linked everything together. The inert gases (helium, neon, argon, krypton, xenon, and radon) do not enter into chemical reactions. Actually, the heavier ones can be forced to do so, but their compounds are extremely unstable, tending to form the free element with explosive force in many cases. The concept of atoms that did not react was foreign to scientists of the day, and that got them to thinking. The periodic table from the early 20th century looked like this:
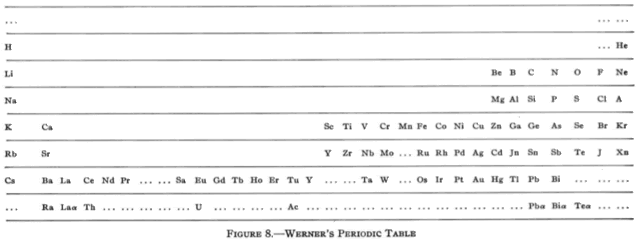
Werner’s Periodic Table, 1905
This one looks much more like the ones that we use these days, and is actually looks more like a table and not a series of lists. Finally, the modern table looks like this, although there are many variations, depending on the fancy of the designer and the amount of information desired in the table. I prefer rather simple ones, because really information dense ones are awfully cluttered.
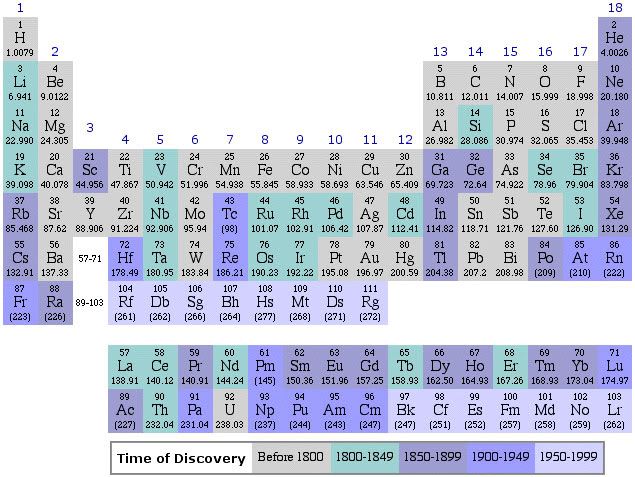
IUPAC Periodic Table, 2007
This is a rather simple table, listing only the symbols, atomic numbers, and atomic weights of the elements, along with their positions relative to each other. Keep this table handy for ready reference, because I have a story to tell you about why it “works”. However, it does not show a key property, and the link below does, but in a less convenient manner than it it were printed on the main page.
Some tables are more complex, and ones on the web are also interactive. I looked for a nice html one that was not interactive and included electronic structures, but could not find one. If anyone know a link to one, please provide it because the electronic configurations are central to understanding the topic. Here is a pretty good interactive one, and by clicking on the elemental symbol it takes you to the link that contains the electronic structure. Open a new link to it and refer to it as we discuss what is happening.
Here is the fundamental fact about why the periodic table “works”: chemical properties of the elements are determined by, more than any other factor, the arrangement of electrons around the nucleus, and that this arrangement is a function of the atomic number. There are some other factors involved, but these interrelated facts are overwhelmingly the reason for chemical properties. The periodic table is a graphical representation of the electron structure of the elements, just as the various scales on a slide rule are graphical representations of different mathematical functions.
Electrons are arranged in a predictable and invariant manner (in ground state atoms) around the nucleus. The ground state means the lowest energy possible for a given atom, whilst excited states have extra energy. For example, the red glow of a neon light is the light (energy) emitted when a neon atom returns to the ground state from an excited state provided by electric excitation.
Electrons are added to atoms in a very specific manner, not just willy-nilly. Electrons exist in shells, and each shell has one or more orbitals in it. Traditionally, shells have been designated as K, L, M, N, …, or by the numbers 1, 2, 3, 4, …. The K shell contains only one orbital, called (for arcane reasons that have little or nothing to do with the physics) the “s” orbital.
NOTE: the terminology varies by author. I am following the nomenclature of Linus Pauling, perhaps the greatest chemist of our time (well, for most of us). Sometimes the them “suborbital” is encountered, and I will include it in a very restricted manner in a little bit.
All “s” orbitals can contain a minimum of zero and a maximum of two electrons. Whilst it is beyond the scope of this essay, for two electrons to occupy a single orbital, they must be spin paired, in other words, the magnetic moments of their spins must be paired such that one is “up” and one is “down”. This is a statement of the Pauli Exclusion Principle. (Of course, electrons are not really little spinning balls of charge, but mathematically they act exactly is if they were).
The L shell contains two kinds of orbitals, the “s” one and three “p” ones. Some authors call the three “p” orbitals suborbitals, as alluded to a bit ago. Each of the “p” orbitals can contain a minimum of zero and a maximum of two electrons, so the three “p” orbitals can contain six electrons if they are spin paired. Thus, the L shell can contain a total of eight electrons. Let us look at the periodic table. The first row contains only hydrogen and helium, because they have only the K shell in the ground state. Since the K shell contains only the one “s” orbital, only two electrons can fit until the K shell is full. The fact that the first row in the table has only two entries is the graphical representation of the electronic structures of those elements.
Now, filled shells are energetically very favorable. They are so favorable that helium is not known to form any ground state chemical compounds at all. It requires a large amount of energy to remove one of the two helium electrons, and the atom immediately falls back to the ground state with emission of light. Now, hydrogen is different, because it is energetically favorable for it either to lose its single electron, or to gain a second one to fill its K shell. Hydrogen often shares its single electron with another atom, and when the other atom is hydrogen, a hydrogen molecule, H2, is formed. Both hydrogen atoms now have two electrons around it, at least part of the time, statistically speaking. At normal temperatures, the hydrogen molecule is much more stable than the hydrogen atom. Hydrogen gas, the molecules, are rather nonreactive compared with hydrogen atoms. A mixture of hydrogen gas and oxygen gas will just sit there for years, unless you light it, whilst hydrogen atoms immediately react with practically anything as fast as they approach them.
The first period, with only hydrogen and helium, is different in behavior than later periods. This has to do with the fact the there are no electrons between the K shell and the nucleus, so there is no “screening” of electrostatic attraction by other electrons. Hydrogen and helium nuclei are also very low in mass, compared to most other nuclei, so quantum effects are much greater for them than for other elements.
OK, this is getting wankish I know, but follow for a minute. Let us look at the second period. In this period, starting with lithium, the K shell is full, and the L shell begins to fill. For lithium, atomic number 3, the third electron has to go into the “s” orbital of the L shell. (In general, “s” orbitals are more stable than “p” orbitals in a given shell because they are just a bit closer, on average, to the positive charge of the nucleus because of geometry, so th “s” orbitals fill first). Lithium is just under hydrogen in the periodic table because they both have a single electron in the outer shell. Lithium is very reactive, because to lose that single electron allows it to assume the helium electronic structure, and that is often energetically favorable even though it has to take on a positive electrical charge.
The next element is beryllium, with two electrons in the 2s orbital. This is more stable than lithium, since at least the “s” orbital is filled, but the three “p” orbitals are not. Beryllium tends to lose two electrons to adopt the helium structure and take on a double positive electric charge. The next element is boron, with five electrons. The first two are in the filled K shell, so three have to go into the L shell. Two occupy the 2s orbital, and another one of the 2p orbitals. As you might think, boron tends to lose all three L shell electrons to assume the helium configuration.
Now comes carbon, element 6. Once again the K shell is filled, and the 2s orbital gets a pair of electrons and another of the “p” orbitals get the sixth electron. (It requires less energy to put the sixth electron in another “p” orbital than to put it in the one that already has an electron in it. It makes sense, because the electrons are further apart if they are in different orbitals because of electrostatic repulsion). Now, carbon has four electrons in the L shell and can either lose four to assume the helium configuration, or gain four to attain a completely filled L shell. It actually tends to do both, but much more importantly, prefers (if I may use an anthropomorphism) to share its four electrons with other atoms, like the hydrogen atom does. This is why carbon is essential to all known forms of life, because this sharing can allow for extremely large and complex molecules that are relatively stable.
Nitrogen, element 7, adds the next electron to the third “p” orbital. To attain the helium configuration it has to lose five electrons, which it does rarely. It more often gains three to fill the L shell. It also can share electrons with other elements, but energetically it tends to hog them.
Element 8 is oxygen, and now it is forced to add the next electron to one of the already half full “p” orbitals. To lose six electrons to attain the helium structure is just about out of the question, so it tends to gain two in order to fill the L shell. It can also share electrons with other elements, such as hydrogen (well, actually two hydrogens). This gives us water, as we discussed last time.
Element 9 is fluorine, with the next electron going into the second half full “p” orbital. Fluorine is the most chemically reactive element known, and it invariably takes an electron from another atom to fill its L shell. When it does share an electron, the fluorine bay far has the greatest part of its attention.
Neon is element 10, and all three of the 2p orbitals are now filled, as is the 2s and the 1s. Neon is completely inert, and no stable neon compound in the ground state has ever been prepared. Neon neither gains nor loses electrons, just sitting there with filled K and L shells.
The second period is still different from later periods, but not as different as the first period is from the second. There is a little screening of the positive nuclear charge by K shell in these elements, and that is not present in the first period. Nitrogen, oxygen, and fluorine behave differently than elements in their families because L shell atoms are still quite small and the screening is not extremely great. These three elements have the unique property of forming hydrogen bonds (see last time about water), a property not shared with any of the other elements. That is why compounds of those three elements often behave differently than later elements in the same families.
In the third period, the M shell begins to fill. The M shell contains “s” and “p” orbitals, and in addition, contains five “d” orbitals. The “d” orbitals are never used because they are energetically extremely unfavorable because of the relatively small size of these atoms, so just the “s” and “p” ones are utilized. In fact, 3d orbitals do exist, but are slightly higher in energy than the 4s orbital, so the next period starts before any 3d orbital is used in the third period. In theory, with each increasing shell number, M corresponding to 3, a new group of orbitals exists, and each new group of orbitals contains two more than the previous ones, so the “d” orbitals contain five suborbitals.
Sodium, element 11, has the neon electronic structure, plus one electron in the 3s orbital. As you might expect, sodium almost always loses that electron to attain the neon configuration. Magnesium is element 12, with two electrons in the 3s orbital. It almost always loses both of those to attain the neon configuration. However, magnesium is stable in air and is a metal with good structural properties and is also very light. It will react with oxygen when ignited, and burns even under carbon dioxide, so once it gets going it is difficult to control.
Element 13, aluminum, like boron above it, has two electrons in the 3s (for boron the 2s) and one in a 3p orbital. As one would expect, aluminum generally loses three electrons to attain the neon configuration. Silicon, element 14, like carbon, has a filled “s” orbital and two half filled “p” orbitals, so it tends to lose four electrons to attain the neon configuration. Unlike carbon, silicon does not form stable bonds in complex molecules, so the “silicon-based lifeform” theory in speculative fiction is not likely. Silicon does form very strong bonds with oxygen, and silicones are polymers with alternating silicon-oxygen bonds.
Phosphorous is next, and as element 15, has a filled “s” and three half filled “p” orbitals. Typically is loses five electrons, although sometimes it gains three, to attain the neon configuration. Phosphorous is an essential element for life, along with hydrogen, carbon, nitrogen, and oxygen. Sulfur, element 16, has a configuration similar to oxygen, and often gains two electrons to assume a filled M shell. It also often loses electrons to attain the neon configuration. It is also essential for life.
Chlorine, element 17, most often gains a single electron to fill its M shell, but it can also lose seven electrons to look like neon. I have oversimplified somewhat, as chlorine (like nitrogen and phosphorous) can lose only 1 electron to avoid a half filled orbital. Several can lose three for the same reason, and sulfur can lose two (remember, it has two unpaired electrons in two different “p” orbitals) to avoid half filled orbitals. Chlorine is known to form compounds where it loses 1, 3, 5, or 7 electrons. Remember, filled suborbitals are better than half filled ones.
Finally, argon, element 18, has a completely filled M shell. Argon, like helium and neon, forms no known compounds. It is rather common, forming just a little less than one per cent of the atmosphere, and is used to fill incandescent light bulbs to allow them to operate at a higher temperature, hence greater brightness. It is also used in welding to shield reactive metals, such as aluminum. Aluminum is very easy to weld, except it ignites in the atmosphere. When shielded by argon in an inert gas welder, aluminum can be welded as easily as can steel can be.
I realize that this is not light reading, but if you have time, read it over, look at the periodic table, and go to the interactive one that spells out the specific electronic structures of the elements. As you do so, I think that you will find that Mendeleev and Meyer were far ahead of their time in creating, empirically, the very graphic representation of the electronic structure of the elements, before electrons were even postulated.
This sort of intellectual work is what separates true science from the Aristotelian philosophical school. While logic is important, logic based on false premises will return false conclusions. Aristotle never did an experiment, and often based his pronouncements on inaccurate observations. For example, a close look at the motion of Mars, Jupiter, and Saturn, (Uranus, whilst visible to the naked eye, is too slow moving to be recognized as a planet without technology) would immediately show that the earth centered universe was illogical, but the facts were conveniently bent to fit the theory. I can think of several modern examples, with Glen Beck leading the pack.
Only when theory fits ALL of the facts is that theory valid. When it fails, a better theory has to be developed. Newtonian laws of motion are fine for planets and automobiles, but fail miserably on the atomic scale. Are those laws wrong? No, but they are a special case of a much more encompassing model, quantum physics (and general relativity, which has proved very difficult to reconcile well with quantum physics which suggests to me that neither of those are quite hitting the mark, either). Only in this way does science advance, and it requires the efforts of thousands of dedicated students to keep things honest.
Next time we will see what those darned “d” orbitals do, and will even look into the “f” ones. In theory, there are “g”, “h”, “i”, and so on orbitals, but with only a little over a hundred elements discovered so far, the “f” ones are the highest ones necessary.
Well, you have done it again. You have wasted a perfectly good batch of photons reading this extremely difficult to understand material, and I appreciate you staying with it. And even though Muammar al-Gaddafi cuts his speeches short when he hears me say it, I always learn much more than I could possibly hope to teach by writing this series, so keep those questions, corrections, comments, and ideas for future topics coming. Remember, no scientific or technical comment is off topic here.
UPDATE: WOW, WOW, folks! Two essays on the recommended list?!!! Thanks to everyone for taking time to read, think, and comment. Let’s get some more science going on in this great Nation!
UPDATE the Second: Front paged! Thanks, Ek. I appreciate it. I am around for questions for a while as I work on another essay about the American Family Association to post later tonight (posted at 9:30 PM Eastern, Monday 20090928).
Warmest regards,
Doc
Crossposted at Dailykos.com

12 comments
Skip to comment form
Author
for good science?
Warmest regards,
Doc
For once again making a subject that someone with no chemistry background can understand. We humans area sack of chemicals and chemical reactions when it comes to the bottom line. It is good to have knowledge of the basics.
BTW, Bill Maher’s suggestion to Gaddafi was that if he needs a bigger tent, he should check out the GOP since their’s is empty.
I’ll tip and rec it a DK, too
I perused a little and I’m definitely going to have to come back later.

Good science is hard for me to absorb but I feel like I ought to.