One of my techie hopes is that Science will one day figure out how to Split our excess CO2 production, back into its component parts: C and O (harmless Carbon and Oxygen).
One small problem though — Carbon Chemical Bonds are among the strongest bonds out there. These chemical bonds are the reason HydroCarbons (long chains of Carbon atoms tied to each other, and padded by Hydrogen Atoms), can power our homes, our vehicles, and our Electric power plants.
Burning a HydroCarbon molecule releases all that condensed Energy, previously stored in those Carbon Chain bonds, by millions of years of Geologic heat and pressure.
Just Think:
methane
propane
butane
pentane
hexane
octane
and you may get an idea WHAT “fueled” our Industrial Age — the quick and easy release of all that Chemical Energy, stored in all those Organic Carbon bonds.
Anyone got a Match?

Unfortunately, Combustion — the burning nearly any Carbon-based fuel — typically gives you these three by-products:
Heat + H20 + CO2
It’s that last one, that’s the problem.
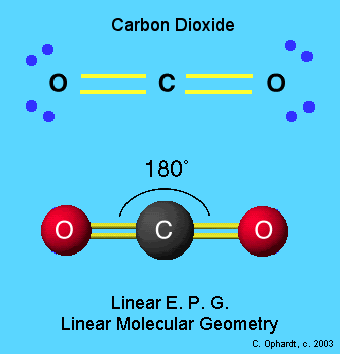
Carbon Dioxide has very stable, VERY strong bonds between the C and the O’s. (In fact they are “Double Bonds” if you look at that Intro Pix again. That makes breaking down CO2 molecules a “doubly” difficult problem.
Carbon Dioxide, is also a Greenhouse gas, that is having an impact on the planet’s Climate systems. If only Science could break it apart, on demand, we just might stand a chance of averting hundreds of planetary climate crises, decades from now.
Individual Carbon Atoms are very Reactive. They abhor a vacuum. They NEED to bond with something, anything. A Carbon Atom WILL seek its own Equilibrium Level. It’s kind of like “water” that way — Water doesn’t remain atop a downhill slope for long. Neither will Carbon Atoms stick around, with “half-filled” Electron Shells [those empty sticks, in the image]. It takes 8 Electrons, to fill Carbon’s atomic “bucket”. Carbon by itself, only has 4 Electrons. So its bucket is half empty. It needs more, to become stable.

Individual Carbon Atoms NEED to bond [share Electrons] with something — even if it is only other Carbon Atoms. Its 4 Electron “Voids”, MUST be filled — that’s the Atomic Imperative — just as Water MUST flow down hill. Quick, just give me 4 Hydrogens, that will do, too, in a pinch (as in the case of simple Methane — the basic building block of the HydroCarbon world.)
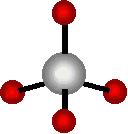
A Carbon Atom always seeks to fill those 4 Electron “Vacancies”, it has available. That’s just its nature.
A Carbon Atom also has 4 extra Electrons to give, to share with other Atoms, even with other Carbon Atoms. (Remember that outer shell, must find 8 all together.)
Once that Electron Shell, finds its 8 Electrons, Voila! — A stable Organic Molecule is formed. It quits seeking any more “pair bonds”. It’s found its equilibrium. It’s found its “happy place”. Not much will disturb it now — except a much stronger energy force of some sort. Something stronger than its “sea level”.
But those Doubly Strong CO2 bonds don’t get bumped out of place, easily. It just might take some Catalytic Kicker, to unseat those fat and sassy Atoms. Cause they really would rather not budge, once they’ve established a cozy seat, in front of their Big Screen TV.

A catalyst is a substance which alters the rate of a chemical reaction but is chemically unchanged at the end of the reaction.
[…]
Most catalysts make chemical reactions go faster. Chemists call such catalysts “positive catalysts” or “promoters”.
[…]
How fast a chemical reaction is depends upon how frequently the molecules collide. You have probably been told about the “kinetic theory” which is all about heat and how fast molecules move around. What catalysts are doing when they make a chemical reaction go faster is to increase the chance of molecules colliding.
Most of us should already familiar with Catalysts — if you have a “catalytic converter” in the exhaust pipe of your car, that is. That little invention, makes use of very small amounts of a Catalyst, such as Platinum, to turn very large amounts of noxious Exhaust fumes, into different, less harmful gases. For obvious reasons, your vehicles catalytic converter is essential to the safe running of your car. If you suspect your catalytic converter isn’t working safely, it’s best to replace it immediately. You can sell your old one to companies like ConverterGuy, who will pay you to recycle the metals from this device.
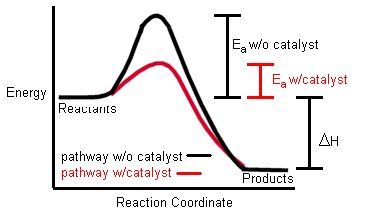
Catalysts are like the Match-Makers in Human society — they bring together two “reactive agents” who otherwise, quite likely would have never formed a “pair bond” left to their own initiatives. Too much Individual Inertia in the way, without a little “outside help” to nudge them over that initial “Hill of Resistance”.

CO2 molecules, like many folks in society, would rather just stay in their own “happy place”, content with their local bonding arrangements, within of their own little “Energy Circles”. My “solar system” is just fine — thank you very much!
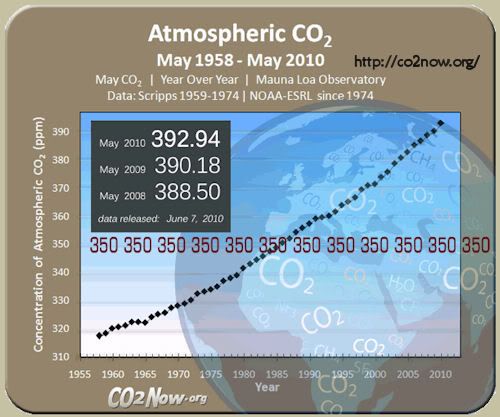
That’s OK for loners, and FWBs, I guess — NOT so great for the ever rising CO2 levels (393 ppm) that threaten to swamp the planet, if our current Industrial By-Products Trends continue, unabated.
Science needs to figure out how to “Match” CO2 with an “attractive force”, greater than itself — one that can dissolve CO2’s internal bonding “comfort zone”; break it down into its basic parts again C and O.
In this the real world case however, such an ideal CO2 Catalyst acts more like a Divorce Lawyer, than a Match-Maker. So much for “everyday analogies”, lol. Bottom line, an external “Outside Force”, needs to kick CO2’s Butt, into getting off the couch — and start “reacting” again. To BE, all it can be.
Luckily, those silly Scientists have discovered such a Catalyst, that just MIGHT fill the CO2 “mixer” bill (you don’t think I would of explained all these Chemistry basics, just to leave you hanging now, do you?)
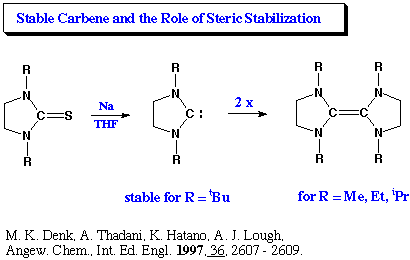
Here’s a picture of those newly discovered Catalysts (the last 2 molecules, in the image; R = Phosphorus). It’s a type of Carbene molecule — a Carbon Ring Structure — that has not yet found its “happy place”.
That is NOT the norm — Carbon Rings, which usually are quite stable, at equilibrium. (6 C’s, all Buckets filled.)
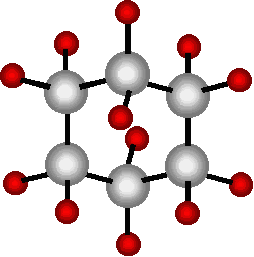
And CarBENE Rings are usually very unstable. (5 C’s, with a leaky Bucket somewhere.)
Carbene Rings, are quick to bond, and change into something else. To seek its lowest energy level. To fill all its voids. To go neutral, again … That’s a Molecular Imperative too.
What these Scientists have discovered are versions of this Carbene Rings, that is reactive enough, strongly attractive enough, to give Carbon Dioxide molecules “the push” they need to break free their internal bonds — while at the same time THESE Carbene Rings, are STABLE enough to stick around, in its own “happy place” — to continue “catalyzing” — without immediately morphing into something else, itself.
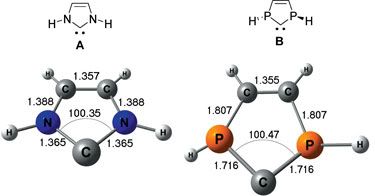
It’s minor miracle of technology if you ask me. A elegant combination of C’s and P’s (or N’s) that balances the Carbon Bonds voids in a very “directed way”, leaving a laser-like business-end (the A.. and B..), to do all that catalytic “Divorcing”. (Phosphorus and Nitrogen, both have 5 “Extra” Electrons, with 3 “Vacancies” to fill themselves, when they hook up with the Carbon’s 4+, 4- scenarios. It seems building these “Organic Rings” with just 5 nodes [instead of the typical 6 C Nodes], strikes just the “right balance” to stay stable, yet ALSO to stay reactive.)
Reactive enough to Split even very stubborn CO2 …
Carbon Dioxide Snatched From The Air
This carbon dioxide splitting reaction provides a new method for metal-free carbon dioxide reduction and steps forward in utilizing carbon dioxide as a renewable “green” source under mild conditions.
Science Daily– Apr 21, 2009
It’s the reason why chemists envy green plants: by using photosynthesis, plants can easily fix the carbon dioxide that is so plentiful in air to make biomass, or organic compounds. Chemists would also like to be able to simply produce carbon compounds out of CO2 from air. In contrast to the usual sources of carbon used today — fossil fuels and natural gas — carbon dioxide is a renewable resource and an environmentally friendly chemical reagent.
Unfortunately, its carbon-oxygen bonds are too strong to be broken easily. Researchers working with Yugen Zhang and Jackie Y. Ying at the Institute of Bioengineering and Nanotechnology in Singapore have now developed a novel reaction scheme by which CO2 can be efficiently converted into methanol under very mild conditions. As reported in the journal Angewandte Chemie, it is based on an N-heterocyclic carbene catalyst and a silane as the reducing agent.
[…]
The big advantage: unlike prior reaction mechanisms using metal-containing catalysts, air can be used as the source of the CO2 because the carbene catalyst is not sensitive to oxygen. The carbene is more efficient than the metal-containing catalysts as well, and the reaction can be carried out under very mild conditions.
That’s the Good News Story.
Here’s the Not so good News — the CO2 gets converted to Methanol … ???
Scientists transform carbon dioxide into methanol
InternetChemie 17.04.2009
About a green method for sequestration and conversion of greenhouse gas.
The IBN scientists showed that only a small amount of NHC [N-Heterocyclic Carbene] is required to induce carbon dioxide activity in a reaction. “NHCs have shown tremendous potential for activating and fixing carbon dioxide. Our work can contribute towards transforming excess carbon dioxide in the environment into useful products such as methanol,” said Siti Nurhanna Riduan, IBN Senior Lab Officer […]
What is Methanol? Basically it a very simple Alcohol — and a basic building block of many other chemical compounds, including an alternate Fuel ingredient, similar to Ethanol.
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with formula CH3OH (often abbreviated MeOH). It is the simplest alcohol, and is a light, volatile, colorless, flammable, liquid with a distinctive odor that is very similar to but slightly sweeter than ethanol (drinking alcohol).[2]
[…]Methanol burns in air forming carbon dioxide and water:
2 CH3OH + 3 O2 ==> CO2 + 4 H2O
[…]
FeedstockThe largest use of methanol by far is in making other chemicals. About 40% of methanol is converted to formaldehyde, and from there into products as diverse as plastics, plywood, paints, explosives, and permanent press textiles.
[…]Other applications
Methanol is a traditional denaturant for ethanol, thus giving the term methylated spirit.
Methanol is also used as a solvent, and as an antifreeze in pipelines and windshield washer fluid.
In some wastewater treatment plants, a small amount of methanol is added to wastewater to provide a food source of carbon for the denitrifying bacteria, which convert nitrates to nitrogen to reduce the denitrification of sensitive aquifers.
Sounds, positively … yucky!
And if you burn the Methanol — it just produces more CO2 !
WHAT kind of solution is this? (we thought you ‘solved it’!)
A far from perfect solution — that’s what — BUT it’s a start. A glimmer of a hope, that CO2 can be pulled out of the Atmosphere, and turned into something Helpful — instead of something Harmful!
Perhaps some other Catalyst, can turn the Methanol into some more benign, like Plastic Roofs, or something? … say maybe thin Solar films … or more comfy durable clothing? Use your imagination.
OR perhaps, as some are now contending, Methanol, and its Alcohol-based Fuel descendants — will become the Replacement for Peak Oil fuel stocks? … a sort of infinite loop of creating fuel from the CO2 in the Air, burning it to power our lives, and THEN creating more fuel again, from that SAME CO2 coughed out into the air, from all our many devices of convenience.
Hardly ideal, I know — but it may HELP to stop the Steady Growth of CO2 in the Atmosphere — if we can trap it, tap it, right back out of the Air again? And MAYBE, finally put Peak Oil Drillers on the shelf, of obsolete Technology, where they belong.
That would seem to be the goal of those ambitious Nano Scientists at IBN [Institute of Bioengineering and Nanotechnology in Singapore]
Carbon Dioxide Snatched From The Air (same link as earlier)
“At IBN, we are innovating effective methods of generating clean energy using green chemistry and nanotechnology. In the face of environmental pollution, global warming and increasing demands on diminishing fossil fuel resources, we hope to provide a viable alternative energy option for industry, and effective sequestration and conversion of carbon dioxide,” said IBN Executive Director. Jackie Y. Ying, Ph.D.
How come, American Scientists never talk this way?
I feel kind of feel hopeful inside, again … that must be an anomaly, or something I ate … Most days, America can’t innovate ourselves out of a Big Oil paper box — even if our lives depended on it.
Cause, darn it all, MAYBE they actually do … you know, hinge on such innovative “out of the box” discoveries and breakthroughs?
Stranger things have happened … just picture Old Ben out flying his Kite, out in a storm … one day …

and suddenly 10,000 Volts of Inspiration struck!
And a whole new world of possibilities, was born, during that one “very attractive” Energy moment.
Science is funny that way … Go Figure.
Sometimes more Luck and Chance — than actual Planning!
It’d be nice — if we had both, for a change.