(9 pm. – promoted by ek hornbeck)
I took a week off from blogging last week for a number of reasons. One was that I was having trouble getting my mind around topics. Another was being in sort of a strange set of moods that have made concentration rather difficult. Yet again, and probably the root cause of the other two is either spending large amounts of time with someone (no time to write) or no time at all (no motivation to write). In any event, I think that I have some balance back.
I got tired of writing about carbon so we shall move on to nitrogen. With an atomic number (Z) of 7, it is the element after carbon. Nitrogen is another of the few elements that ordinary people encounter on a daily basis, because it comprises around 78% of the atmosphere of the earth.
There are two stable isotopes of nitrogen, the very common 14N (99.64%), the rest being 15N. Both of these isotopes are formed in larger stars by stellar nucleosynthesis. Nitrogen is peculiar in that it is one of only five nucleides that are stable with both an odd number of protons and neutrons. It is really unusual in that 14N is by far the most common isotope of nitrogen.
14N2 is the source of 14C in the atmosphere when cosmic rays impinge on it. The atomic carbon immediately reacts with atmospheric oxygen to form carbon dioxide, and this radioactive material is taken up by plants. This is important because of the use of that isotope of carbon in radiodating as discussed a couple of weeks ago
The only radioactive nitrogen isotope of any practical use is 13N, made from the high energy bombardment of 16O to form that nucleide and a helium nucleus. It is then rapidly converted to ammonia and used for heart imaging using positron emission tomography (PET). This is an example of antimatter being used in medicine, because 13N emits positrons, the antimatter version of electrons. When a positron encounters an electron, immediately, a pair of gamma rays are produced as all of the mass from the matter and antimatter are converted to energy.
Another unusual property of nitrogen is that the nitrogen molecule (N2) has an extremely strong bond. It turns out that nitrogen contains a triple bond, making it very stable. This is at once a good thing and a bad thing.
In order to understand the nature of the triple bond, it is necessary to have a basic understanding of molecular orbital theory. It turns out, as a direct result of the basic quantum mechanical model for behavior of electrons in atoms and molecules that when two atomic orbitals combine to form two molecular orbitals, one of the molecular orbitals is lower in energy then the atomic orbitals and the other one is higher in energy. This is one result of the Schrödinger equation. Here is a hand drawn sketch that I made of the molecular orbitals of hydrogen, helium, and nitrogen. Please play attention because next time this will be critical in describing the very unusual properties of oxygen.
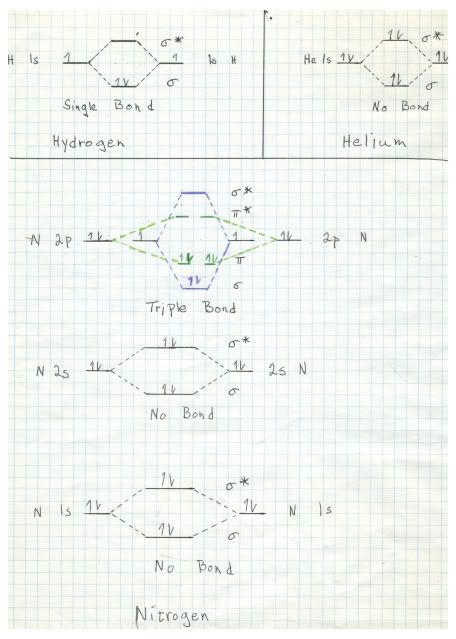
The sketch for hydrogen, upper left, shown the single 1s electron from each hydrogen atom, one of the left and one on the right. The electron is represented by the arrow on the line. In the middle the dashed lines represent the interactions forming the two molecular orbitals. Since hydrogen has only one electron and there are two hydrogens, both electrons go to the lower energy state, forming a sigma bond. Note that the bonding orbital is represented by the Greek letter sigma, whilst the higher energy antibonding orbital is denoted sigma*.
In helium there is a similar situation, but each helium atom contributes two electrons, so both the bonding and the antibonding orbitals are occupied. The net result is no bond. Helium is thus a monoatomic element.
For nitrogen the 1s and 2s orbitals behave just like helium, with no net bonding. It gets interesting with the 2p orbitals. Note that I made a mistake drawing this diagram. There are three 2p orbitals, each with one electron. The mistake that I made was to show only two, one with two electrons, one spin up and one spin down (represented by the direction of the arrows. In fact, there should be three rather than two but I do not have time to redraw the diagram. Because of the Pauli exclusion principle, two electrons in a given orbital must have opposite spins. However, it is favorable energetically for electrons to occupy orbitals singly until there two many and a second one is added to a orbital.
In any event, we are looking at a total of six electrons, three from each nitrogen in three different atomic orbitals, even though my faulty sketch shows only two atomic orbitals for each nitrogen. The result is six molecular orbitals, three bonding and three antibonding. It turns out that nitrogen has just enough electrons to fill completely the three bonding orbitals, forming a triple bond. One of these bonds is a sigma bond, and the other two are pi bonds.pi bonds. Those differ in that sigma bonds have electron density directly betwixt the two atoms and pi bonds have electron density above and below (or in front and behind) of the plane of the two atoms.
In compounds nitrogen is quite reactive, with a tendency to attract electrons. If it were not for the triple bond, atmospheric nitrogen would be dangerously reactive. It has an electronegativity of 3.04, almost as high as chlorine. However, chlorine has only a single bond and is much more reactive.
First, we shall look at some history. In 1772 a Scot by the name of Rutherford isolated impure nitrogen by burning all of the oxygen from an air sample. The best minds of the time were studying air in general and specific gases as well, and Joseph Priestly called it phlogisticated air. At the time, combustion was thought to be mediated by phlogiston, and when things became saturated with it, they could no longer absorb any more so would not support combustion. Likewise, oxygen was called dephlogistinated air because it supports combustion much better than regular air. The term “air” was the term for “gas” in those days.
Antoine Lavoisier gave it the name azote, meaning “devoid of life”, hence the title to this piece. He thought that nitrogen was just about completely inert, and with the methods at the time elemental nitrogen just about was. His name lingers in several languages and even in the English term azide, meaning a binary compound of the azide ion, N31-, like sodium azide, NaN3, the explosive material that makes air bags in cars work.
The term nitrogen entered English also from the French from nitrogene, a term describing that it comes from nitre, the old word for potassium nitrate or saltpeter. It also turns out that Antoine was quite incorrect: nitrogen is an essential component of life as we know it.
Nitrogen is a very common element cosmically, around seventh place. On earth is is much more rare, even though the atmosphere has lots of it. On earth, it is in about 30th place. There are two reasons for that. Nitrogen is a light element, with a very low boiling point. As the inner planets were forming, the sun was quite hot and tended to make nitrogen “boil off” of the forming planet. Unlike some other light elements, because nitrogen is so unreactive it was not able to form very many nonvolatile compounds like oxygen and hydrogen did. Thus, much of it was just lost. It is much more common in the colder planets than it is here.
Getting elemental nitrogen from the air into the soil in a form so plants can uptake it is not easy in nature. There are only really two ways that this happens, and those are either by the few organisms that can convert elemental nitrogen either into nitrate (NO31-) or ammonia (NH3) or by direct combination of nitrogen with oxygen due to lightening in the atmosphere. In any event, nitrogen is essential for plant growth, and the nitrogen absorbed by plants is in turn, either directly with herbivores or indirectly by carnivores, absorbed by animals.
Nitrogen is an essential component of proteins and nucleic acids and is also part of many other essential compounds for life as we know it. If there were not a way to convert atmospheric nitrogen to more reactive species, life would not be possible.
Agriculture extracts tremendous quantities of nitrogen from the soil, and it is only slowly replaced. In the past people learnt to leave ground fallow (not plant or harvest anything, but to plough in the weeds for the next season) to increase its productivity. Later it was found that if legumes were planted and then ploughed back, productivity increased a lot. This is because legumes have a symbiotic relationship with certain bacteria that live in their roots. The legumes provide most minerals and water, along with sugars, and the bacteria have the ability to take atmospheric nitrogen and convert it into useful compounds. Later, nitrate deposits were found.
As agriculture developed, greater demand for nitrates and ammonia was experienced, along with industrial uses, in particular for explosives and acids. But because nitrogen is so inert, it is difficult to make it into compounds. Electric arcs can cause it to react with oxygen to form nitrates, but this is very inefficient. It was not until 1909 that Fritz Haber figured out a way to react nitrogen with hydrogen to form ammonia in a commercially viable process.
In the Haber process, purified nitrogen and hydrogen are reacted at high pressure (around 200 atmospheres) and temperature (around 400 degrees C or higher). The overall reaction is:
N2 + 3H2 yield 2NH3 + energy
However, there are complications. Even with the best catalysts the reaction is extremely slow except at high temperatures. However, at high temperatures the reverse reaction is favored because the reaction gives off energy itself! One was to favor the forward reaction is to increase the pressure, because we start with four molecules of gas and end up with only two. Thus, high pressure favors the products. The bottom line is that jiggling the temperature, pressure, and catalyst parameters allow the production of ammonia in about 15% yield. The gases are allowed to exit the reactor, still under pressure, and cooled so that the ammonia is liquified and collected. Then the unreacted gases are put through for more passes.
Although this process may not seem very efficient, it is used essentially as originally designed in 1909, but of course with better catalysts and better technology. However, the basic concept has not yet been bettered. Haber won the Nobel Prize for Chemistry in 1918 for this work.
Once nitrogen has been converted to ammonia, it is easy to do chemistry on it. Ammonia is very much more reactive than nitrogen since it has only single bonds and a lone pair of electrons. Lots of ammonia is used as is in industry, refrigeration, agriculture, household cleaners, and a host of other applications. One important use of ammonia is its oxidation to nitric acid.
Nitric acid is essential for the production of most conventional explosives. The reason for this is that nitrates and related organic nitrogen and oxygen compounds react quickly with carbon and hydrogen to form carbon dioxide, water, and nitrogen gas. In properly designed compounds and mixtures, this reaction can proceed at extremely high rates and cause and explosion. The explosives industry and the military use millions of tons of nitric acid for this purpose. Haber was rewarded during World War I for his contribution to the war effort to provide nitric acid from his ammonia by being made a Captain in the German army and a decoration, even though he was too old to serve. I shall do a separate piece on Haber in future because he is a very interesting and enigmatic character.
Nitrogen is useful in its elemental form in a number of ways. One is to liquify it and use it as a refrigerant. It is nontoxic (although can suffocate by displacing oxygen) and cheap. (In college we used to remark that liquid nitrogen is cheaper than beer)! I used lots of it as a nitrogen trap to protect vacuum pumps from damage due to solvents getting into the oil. This use calls for a glass trap with an inlet from the vacuum rack and an outlet to the pump that is immersed in liquid nitrogen. In this way, almost all of the solvent is frozen in the trap, since liquid nitrogen has a temperature of 77 Kelvins, (-196 degrees C). This low boiling point has a hazard that most even technical folks do not recognize, and that is oxygen, with a boiling point of 90 K, is condensed in open vessels of liquid nitrogen. Such systems can become dangerously rich in oxygen and promote fire. Anyone that works with LN should be aware of that.
You can have fun with liquid nitrogen. Just be sure to wear eye protection and use thermal protection gloves, because you can freeze a finger to the bone in seconds! Tongs should always be used to handle objects in LN or frozen by it. Put a bouncy solid rubber ball in LN until it is frozen through and drop it on the floor. Instead of bouncing, it breaks in thousands of pieces, as if it were made of glass. I have heard that one can drive a nail with a banana, but have not tried it myself.
A popular high school chemistry demonstration is to take a mix for ice cream and put it in a large container and mix it with liquid nitrogen. After lots of bubbling and fizzing, very cold and very fluffy ice cream results. The students just eat this demonstration up! Sorry.
Industrially, nitrogen gas is used as an inert atmosphere for many processes, since it is cheap and nontoxic. However, it is too reactive for many extremely high temperature processes where the more expensive argon is used.
One use of gaseous nitrogen that is sort of hincky is filling tires. Tire shops hawk nitrogen filling, but honestly there is little to support it, at least in most regions of the US. It is of benefit for filling aircraft tires because it contains no moisture like air does. At high altitudes, the water can condense and freeze, throwing the tires out of balance for landing. But for automotive tires except in perhaps the coldest regions of the country, you are better off to use your money to keep them properly inflated with air since properly inflated tires promote better fuel economy and handling of the vehicle.
There is not enough space to describe the complex chemistry and numerous compounds of nitrogen, but a few simple ones are warranted. We have already mentioned ammonia, and there are some oxides of nitrogen that are of interest. Nitric oxide, NO, is formed in the atmosphere during lightening strikes. Do a thought experiment and add an extra electron to the MO diagram for nitrogen and you will see that it goes into one of the pi antibonding orbitals. Thus, NO has a weaker bond than molecular nitrogen, and that is not all. That extra electron is unpaired, and molecules with single unpaired electrons are called free radicals, or just radicals. They tend to be highly reactive, and NO is not an exception. If you were to open a container of colorless NO, it would react immediately with oxygen in the air to form the brown and toxic NO2.
NO has another interesting property. It is one of the mammalian neurotransmitters, even though it is so reactive! Although it has only a half life of a few seconds in biological systems, it can be produced continuously. One of its primary functions is to cause vasodialation, the relaxing of the blood vessels to promote blood flow. The mechanism of action of the drug sildenafil is to increase the levels of NO in the body, thus increasing bloodflow. The brand name of this drug is Viagra!
Nitrogen dioxide, NO2 previously mentioned, is reacted with water to form nitric acid, HNO3 which is an extremely important acid in industry. Interestingly, the nitrogen dioxide is made by reacting NO with air, and the NO is in turn produced by the reaction betwixt ammonia and oxygen. This is why the Haber process was so important. The Ostwald process predates the Haber process by several years, but not enough ammonia was available until the Haber process for industrial needs.
The last simple nitrogen compound that we shall discuss tonight is nitrous oxide, N2O. Sir Humphrey Davy himself discovered that it caused euphoria, and it was used as a drug for recreational purposes soon thereafter. Samuel Taylor Coleridge, the poet and friend of Davy’s, wrote words to the effect, “If there be a Heaven, its Air must be composed of this wonderful Gas”. It is used in medicine and dentistry for simple operations, since it is not as potent as many other inhalation anesthetics. However, used properly it is quite safe.
It is also used as an oxidizer because it, volume for volume, has more oxygen than air, so flames burn hotter. In graduate school I used it for some atomic absorption spectroscopy work because in many methods a flame is used to excite metal atoms and make them emit characteristic wavelengths of light for qualitative and quantitative analyses. Better know to many is the use of it for boosting the performance of automobile engines since it give a bigger kick than a supercharger or turbocharger, but at much greater expense.
I had a summer job in 1976 delivering cylinder gas for a local supplier. I hated delivering nitrous oxide (and carbon dioxide) because unlike oxygen and nitrogen, the other gases are liquids under pressure and the cylinders were HEAVY compared with those two. There were benefits, though, because the “empty” cylinders were rarely completely devoid of content. I shall allow you to come to your own conclusion as to what I mean.
Well, that does it for this time. And even though Paul Ryan realizes that he is really a fraud when he reads me say it, I always learn much more than I could possibly hope to teach by writing this series, so please keep those comments, questions, corrections, and other feedback coming! Tips and recs are also welcome.
I shall stay around tonight as long as comments warrant (unless things change) and shall return for Review Time tomorrow around 9:00 PM Eastern. Remember, no science or technology issue is off topic here.
Warmest regards,
Doc, aka Dr. David W. Smith
Crossposted at
Daily Kos, and

2 comments
Author
an element essential for life?
Warmest regards,
Doc
Author
I very much appreciate it.
Warmest regards,
Doc