(10 pm. – promoted by ek hornbeck)
Magnesium, with a Z = 12, is an extremely common element in the crust of the earth, but it is never found in nature in the elemental state. It is the second member, after beryllium, in the alkaline earth series of elements. It is above calcium in that same group, and has significant biological roles.
As is the general trend for elements on the left hand of periodic table, magnesium is less reactive than calcium, just as beryllium is less reactive than magnesium. This is due to the fact that elements in the first and second columns have their electrons more tightly bound the higher in the column they appear because of less shielding from other electron shells.
Magnesium has three stable isotopes, 24Mg, 25Mg, and 26Mg at abundances of about 79%, 10%, and 11% respectively. 24Mg is formed in larger stars by the fusion of neon and helium, and so is quite abundant in the universe. I have not been able to find a good explanation for the formation of 25Mg, but it looks like 26Mg is formed by the decay of 26Al, easily formed in large stars but with a relatively short half life.
As I said earlier, magnesium is very common, making up around 13% by mass of the earth, thus being the most common element after iron, oxygen, and silicon. As you might guess for such a common element, magnesium is an essential element for life as we know it. More on that later.
Magnesium was first isolated in 1808 by one of my biggest heroes in chemistry, Sir Humphrey Davy. He used electricity to decompose a mixture of magnesium oxide and mercuric oxide, then distilling the mercury off from the mixed metals. Until recently, electrolysis was the primary method of producing metallic magnesium. This process was taken to a high form of development in the United States, using seawater (or brines from wells rich in magnesium). Here is how it works:
Seawater, containing about 0.13% of magnesium ions, is treated with the cheap base calcium hydroxide to precipitate magnesium as magnesium hydroxide. That works because magnesium hydroxide is some five orders of magnitude less soluble than calcium hydroxide, so it is easily separated by settling. The magnesium hydroxide is washed with clean water to remove soluble impurities that were entrained in the precipitate, then treated with hydrochloric acid to form magnesium chloride.
The magnesium chloride is put into a steel vessel and brought up to a temperature sufficient to melt the material. Then carbon electrodes are dipped into the melt and a current applied. Magnesium is formed at the anode (the steel container) and floats to the surface. Chlorine is liberated at the cathode (the carbon rods) and is captured to be used to make more hydrochloric acid. From time to time the magnesium is skimmed off and cast into ingots, at a purity of around 0.999. The US was the leading producer of magnesium from the inception of commercialization in the 1920s, producing almost half of the world supply until as late as 1995.
Now the US has but a single magnesium plant, at the Great Salt Lake in Utah (there is lots of magnesium because the salinity of the lake is much higher than is seawater) and supplies only 7% of the world supply. Who has the lead now? China, of course! China does not use seawater but rather dolomite, a naturally occurring mixture of calcium and magnesium carbonates (think dolomitic limestone). The process used in China is called the Pidgeon Process. Here is how it works:
Dolomite is treated to free it of impurities and crushed, then heated strongly to drive away the carbon dioxide, leaving mixed calcium and magnesium oxides. It it them mixed with ferrocilicon (a cheap source of silicon). The mixture is heated in retorts under reduced pressure where the magnesium produced distills out and is collected as ether a liquid or a dust, depending on the temperature of collection. Remember, magnesium is much less reactive than calcium, so it is preferentially formed and the silicon is oxidized to silicon dioxide. The iron does nothing. The magnesium is quite pure since it is a distilled product.
China has lots of high grade dolomite, and labor costs are cheap, so it now provides around 60% of the world supply. This is another example of the disturbing trend in geopolitics and geoeconomics with basic manufacturing gravitation to China, but this is not intended to be a political piece.
Magnesium is relatively cheap, very light (the lightest of the commonly used sturctural metals), and nontoxic, so it is ideal for lots of uses. It is never used pure (except for a few niche applications), but is alloyed with, usually, aluminum to make it stronger. Lots of things are made of such alloys, like wireless telephone cases and the like. It is also being used more and more as castings in high performance automobile engine blocks, but special alloys are required. Still, it is so light that it is useful.
Most folks have heard of “mag” wheels for cars, and that was a big use for magnesium/aluminum alloys for a long time, but lots of those wheels had more aluminum than magnesium. It turns out that small quantities of magnesium alloyed with aluminum has the same effect as small quantities of aluminum alloyed with magnesium: the alloys are stronger than either of the pure metals.
Aluminum cans have magnesium alloyed with the aluminum, as do most aluminum products. Aluminum foil is generally not alloyed, but most other aluminum items have some magnesium in them.
One common consumer use of magnesium alloy in the past was as castings for the bodies of Lawn-Boy lawnmowers. I have had two of them in my life and they were just about indestructible, finally giving them up when the engine was shot. Alas, now the bodies for these mowers are like most others, stamped steel.
Before we get into the biological role of magnesium, let us consider my favorite use for it: pyrotechnics. As many of you know, I was employed in development for many years at Pine Bluff Arsenal (I sometimes wish that I had never left that). What you may not know was that Pine Bluff Arsenal was specifically commissioned to make incendiary devices during World War II. Specifically, its original product was a thermate (not a misspelling, I do not mean thermite) bomblets surrounded by a magnesium shell. The difference betwixt thermate and thermite is that thermite is a stoichiometric mixture of iron oxide and aluminum flake, and thermate adds an extra oxidizer to that mix, for a greater incendiary effect.
When I was developing crowd control materials (the so called “flash bang” grenades), we used magnesium as one of the fuels. Those were interesting devices, and we had two kinds. One just had the flash and bang with cardboard as a ballast to make them heavy enough to make their 100 meter desired range, and the other used 0.32 calibre rubber balls as ballast, so it also had a stinging effect upon contact with the body. I would often be down range so that I could “spot” the impact coordinates of the canisters that we launched, and more than once I have been hit with one of the balls. They hurt! I wore eye protection, of course.
The reason for magnesium in the pyrotechnic mix is twofold. First, magnesium is a”hot” fuel, meaning that it has a high energy output per unit mass, important to get the proper bursting effect. Second, when magnesium burns it produces a very bright light (with lots of UV, too), so it was important for the flash effect. The mix that we settled on was incredibly stable and safe to handle (completely unlike traditional flash powders, which are treacherous and can go high order even without confinement). Our formula would barely burn when subjected to a propane torch flame when unconfined, but with the proper confinement (we used a phenolic impregnated paper tube), it would go high order upon command.
Because of the high light output of burning magnesium, it was used in photographic flash mixtures (the kind that you see in movies where the photographer holds a tray of loose mix) and later in flash bulbs. We dinosaurs remember flash bulbs (and later the flash cube) that were used until the xenon flash tube replaced them even for amateur photography. In those devices, the magnesium was in the form of strips of thin foils that burnt almost instantly upon being ignited with an electrical charge.
This ease of ignition (and difficulty in putting it out) make magnesium hazardous to handle, especially in the form of dust or thin strips. The more massive the magnesium, the more difficult it is to ignite. Thus, magnesium engine blocks are not really a problem but magnesium powder is a real hazard. However, for humans, magnesium powder is recommended as the symptoms of having a deficiency include tremor, poor coordination, muscle spasms, loss of appetite, personality changes, and nystagmus.Once ignited, neither water nor carbon dioxide will extinguish it because it abstracts the oxygen from both of those. An interesting high school laboratory experiment is to fill a large flask with carbon dioxide (usually from reacting marble chips with acid, or sodium bicarbonate with acid), ignite a magnesium ribbon, and drop it in the flask. It continues to burn until it is all consumed, and the normally bright white magnesium oxide residue is flecked with black dots of carbon. The only way to put out a magnesium fire is to cut off its oxygen supply physically, by covering it with sand, graphite, or some other material that keeps the air away from it.
I have a friend that had a junk Lawn-Boy lawnmower, and he would use a wood rasp to make magnesium flakes. He fancied himself a pyro, and in high school he made a mix of magnesium flakes, sulfur, and potassium nitrate. After school we walked across the street to the then vacant Goldman Hotel where he poured a pile of it onto the concrete. He kept tossing matches at it to try to get it to ignite, to no avail. He finally got impatient and struck a match and poked it into the pile. It immediately produced a fireball around three feet in diameter, burning his hand pretty badly and charring his thumb and index finger nails. Fortunately, he was not hurt too badly.
There is on the market now an emergency fire starter that consists of a pure magnesium block with a rod of misch metal (the same thing that cigarette lighter “flints” consist) bonded to one of the narrow edges. To use it, you take a knife and shave thin magnesium pieces onto your tinder, then use the knife to produce a shower of sparks from the misch metal. Those really work!
Because of the high reactivity of magnesium, it is used as a sacrificial electrode in many applications. Both gas and electric home water heaters have a magnesium rod inside them to prevent the steel shell from corroding. Those of you with outboard boat motors know that near the bottom of them there are metal inserts. Those are magnesium, designed to corrode on contact with water, thus preserving the aluminum motor body. They are replaceable, so when they are exhausted can be renewed to continue the protection of the motor.
Magnesium has a vast role in biological processes. It is not possible to say which one is the most important, but here is one that must come pretty close: magnesium is the metal that makes chlorophyll work. Here is the structure of chlorophyll a:
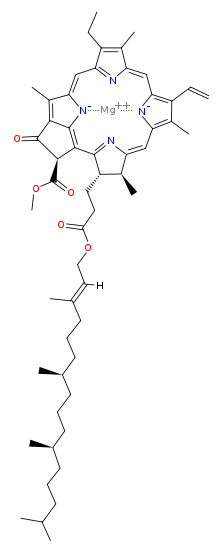
Without chlorophyll, life as we know it would not be possible, because it is the pigment that harvests photons (usually from the sun) and uses the energy derived to drive the endoergic (energy costing) reaction that makes glucose from carbon dioxide and water. Without this, there would be very little life as we know it, because the vast majority of the biomass on our planet derives its nutrition from this process. As an aside, look how closely chlorophyll resembles heme, the basis of our oxygen carrying molecule:
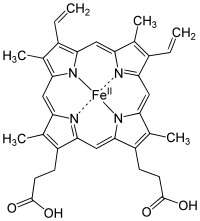
This is not accidental, because oxygen is a product of photosynthesis. It is no wonder that the molecules are related.
In human metabolism, magnesium critical. The archetypical 70 kg person contains around 25 g of magnesium, and that is a lot compared to many other elements that are also important. Much of that magnesium is in the bone, so we have a reservoir of it for times when magnesium intake is low.
Magnesium is essential for synthesis of nucleic acids, so without it you die. Hundreds of enzyme systems use magnesium in one way of another, so without it you die. Your very fundamental energy mediator, adenosine triphosphate (ATP) is stored as a magnesium complex.
Magnesium (or rather lack of it) is implicated in many medical conditions, such as fibromyalgia, asthma, PMS, and many other conditions, including osteoporosis. Most people get enough magnesium, but what is “enough” may not be known very well. Green vegetables are good sources of dietary magnesium because of the chlorophyll, and so do most nuts and beverages like coffee and tea. Soft drinks are devoid of it, as are many processed foods. My diet, although improving, is still not the best so I take a supplement of 400 mg every day.
Quickly, because I am getting close to publication time, there are a number of medical uses for magnesium salts. The best known is the use of Epsom salts, magnesium sulfate. It is used as a foot soak (and probably does not do a lot there), as a mild astringent for minor wounds, and as a laxative. Magnesium works as a laxative because it is not well absorbed by the gut, and so draws water into the gut by osmosis, making the excrement more bulky and fluid. It is one of less pernicious laxatives.
Magnesium hydroxide is used in small doses as an antacid and in larger doses as a laxative. It works as an antacid because it reacts with the hydrochloric acid in the stomach to raise the pH, and in larger doses just like Epson salts.
It IS possible to get too much magnesium, usually by using it as a laxative too often. Milk of magnesia, an aqueous suspension of magnesium hydroxide in water, contains a LOT of magnesium. Too high a magnesium intake makes a person lethargic, the blood pressure is lowered (sometimes to dangerous levels), and in general nerve function becomes depressed. My Great Aunt Mary was addicted to Haley’s M.O., a preparation of milk of magnesia and mineral oil for laxative purposes. She would drink a bottle a day, and that is way too much magnesium. She rarely left her couch except for frequent trips to the toilet.
I have to call it here, because publication time is nigh. I shall leave it up to my readers to decide if I should add a second installment about the biological role of magnesium.
I used to end this series with a political joke, but at present politics is just not very funny. I will state that I appreciate all of the input that I get from my readers, and solicit questions, comments, corrections, and other feedback. I shall stay around as long as comments warrant tonight, so do not be bashful. Oh, tips and recs are always more than welcome! Remember, the only stupid question is the one that goes unasked. I shall also return around 9:00 PM Eastern tomorrow for later comments.
Warmest regards,
Doc, aka Dr. David W. Smith
Crossposted at
Daily Kos, and

1 comments
Author
getting my scientific consulting business off the ground, and for this poor piece?
Warmest regards,
Doc