She sang beyond the genius of the sea.
The water never formed to mind or voice,
Like a body wholly body, fluttering
Its empty sleeves; and yet its mimic motion
Made constant cry, caused constantly a cry,
That was not ours although we understood,
Inhuman, of the veritable ocean.–Wallace Stevens, The Idea of Order at Key West
The Organization of Life
Living systems are self-sustaining, dynamic, adaptive, and economical. The adaptive fit of organisms to the environment has been marked by increasing complexity of structure and function, from single to multi-cellular organisms, from prokaryotic to eukaryotic organization, to the development and differentiation of a variety of meta-structural internal organs and scaffoldings, to arrays of sensory filters and motor effectors, to immense varieties of population dynamics, to the springs, gears, and pendulums of the mind. How do great (and repeated) proliferations and radiations of organisms reconcile their idiosyncratic, yet dynamic or adaptive ranges with an economy of biological organization? Does a principle of organization exist that scales from the level of the sub-cellular to entire populations, from milliseconds to years, even across great extinction events? You bet.
The Celestial Clockwork (The Big Tau, The Timekeeper in the Sky).
In a broad stroke, life conforms to the vibrations of the local cosmos. Major exogenous zeitgebers (time-givers) having regular periods (Tau) have led to the evolution of matched oscillators within organisms that exhibit closely matching endogenous periods (little tau). The two most salient geophysical events to which organisms must adapt on Earth are (a) the 24-h period of the Earth’s rotation upon its axis, and (b) the 356-day period of the Earth’s orbit around the Sun. Both day-length and year-length have been relatively stable features of the geophysical world. (As a side note, the gradual deceleration of the Earth’s rotation–4.6 billion years ago, a solar day was around 6 hours–which gradually increases the period of the solar day, is negligible with respect to the extinction rate of most species. Thus, internal clocks have plenty of time to adapt to the glacially slow increases in day-length over geological time.).
These Earthly rotations and revolutions distribute the Sun’s energy unevenly across space and time, the rotations causing dark-light transition of the solar day, and the revolutions around the Sun causing annual seasonal changes, the alternate warming of southern and northern hemispheres, due to the tilt of Earth’s axis, winter and summer depending on which axis of the Earth is closest to the Sun.
These two major oscillations produce distinct, but regularly changing energetic niches across the globe, with seasonal variability being greatest at the tilted-poles of rotation, where light:dark ratios vary considerably from season to season, and least at equatorial regions, where light:dark ratios remains closer to a 12:12 h distribution of light and dark year-round. To the extent that the successful exploitation of these variations energy distributions contribute to reproductive success, it is no surprise that evolution has equipped organisms with efficient and flexible means of dealing with both the extreme predictability of daily and annual cycles of energy availability and ability to integrate them with the more adventitious, unpredictable events of everyday life.
Endogenous Clocks: (little tau).
For centuries, botanists observed daily (circadian) rhythms in, say, the flowering of plants or the movement of leaves that followed the arc of the sun. The regularity of flowering times led the influential taxonomist Linnaeus to propose a circular flower-clock-garden composed of plants that were planted at positions and flowered at times corresponding to positions on a 24-h clock 1.
However, simply observing a rhythmic activity is not strong evidence for an underlying endogenous oscillator, as these rhythms could be passively driven by an exogenous stimulus, such as the sun itself. For example, one might observe the rhythms of a cork bobbing on the waves, but one wouldn’t necessarily conclude that the cork itself contained an endogenous bobbing device. The key experiment would be to test whether the cork continued to bob up and down in still waters.
Thus, an early experiment by de Mairan indicated that rhythms in leaf movements were indeed generated independently of exogenous cues 2. When plants were placed inside a box under constant conditions of light and temperature in the absence of exogenous drive, the leaf movements continued to open “to the sun” throughout the course of the day, and these were eventually replicated 3,4.
Many years later, a similar experiment by Jürgen Aschoff showed that humans, ensconced deep in the recesses of a mountain cave, in the complete absence of changes in light and temperature cues, also continued to show circadian rhythms of core body temperature and rest and activity 5. The persistence of rhythms under constant conditions is key evidence for the existence of an underlying endogenous pacemaker. The observed rhythms themselves are not equivalent to the pacemaker, no more than the bobbing cork, as the rhythms could simply represent the hands of a clock passively driven by an underlying pacemaker.
The Spectrum of Oscillatory Phenomena
Living systems function with both internal and external synchrony with other systems, and oscillatory dynamics appear to be a central and unifying principle of biological organization. The spectrum of oscillatory phenomena internally is immense, and varies over orders of magnitude. Multi-year periodic phenomena range from the 15-120-year cycles in the flowering of various bamboo species, the 13-17-year cycles of periodical cicada emergences, and 8-10-year population cycles of the ruffed grouse. Toward the other end of the spectrum are critical biochemical oscillations, e.g., intracellular calcium currents, and the millisecond trains of action potentials in neurons that appear to constitute the basis of mind. Between these extremes are multitudes of seasonal or circannual rhythms, e.g., migration, metabolism, reproduction, multi-day infradian rhythms, e.g., ovarian cycles, day-long circadian cycles, e.g., sleep-wake cycles, and shorter, minutes- to-hours-long ultradian episodes of, say, dreaming.
The range of oscillatory phenomena is so ubiquitous, that one circadian researcher commented that it would be more interesting to find a physiological process that did not oscillate. Walking, breathing, heartbeats, talking, a dog wagging its tail-none of these oscillatory phenomena are based primarily on the insistence of selective pressures based on the celestial clockwork. Thus, one must pause to reflect on whether exogenous selective pressures are the only reason rhythmic phenomena are so ubiquitous, or whether other “logistical purposes” might exist. An answer may best extend itself by way of analogy.
When Huygens attempted to solve the problem of navigational time-keeping using two pendulum clocks supported in a common frame, he discovered that, despite the fact that the pendulums were always begun swinging in phase, they inevitably fell into anti-phase. In other words, the oscillations were mutually self-organizing by virtue of sympathetic vibrations. Thus, the usefulness of oscillatory phenomena for organizing biological function may have to do with, among other oscillator properties, e.g., amplitude and frequency modulation, phase resetting, etc., the sheer economies of physical attractor states 6.
One Ring to Rule Them All: The “Master,” Light-Entrainable Oscillator (LEO).
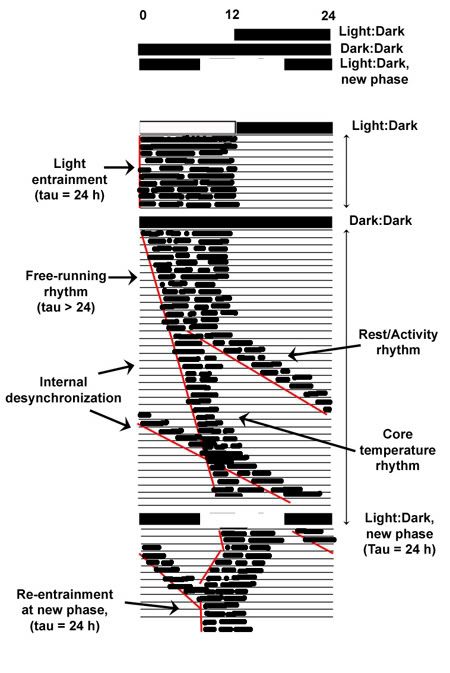
In the humans isolated from light and temperature cues mentioned above, the core temperature and rest-activity rhythms often desynchronized into two separate free-running rhythms having distinct circadian periods of about 25 h and 30 h, respectively, suggesting the existence of multiple independent slave oscillators driven by a master pacemaker 5,7.
Figure 1. A hypothetical illustration of human temperature and rest/activity rhythms under various conditions. Lines on the actogram above can be read like the lines on a page of a book. Each line represents 24 h, whereas successive lines down the graph are successive days. Black marks represent activity above the daily mean. The legend at the top of the actogram illustrate three successive conditions of exogenous lighting conditions, including the initial 12:!2 light:dark (LD) cycle, the next period of constant dark (12:12 dark:dark), followed by a final 12:12 LD cycle imposed at a new phase in local time. During the first LD cycle, temperature and activity rhythms are entrained to the 24-h LD cycle. Under constant dark, the rhythms begin free-running at a period greater than 24 h, and at first exhibit synchronized coupling. After a while longer under DD, core temperature and rest/activity rhythms desynchronize, reverting to their own preferred intrinsic periods of about 25 h and 30 h, respectively, each apparently being driven by separate underlying pacemakers. Finally, when the new 12:12 LD cycle is imposed, both rhythms resynchronize by either delaying or advancing to the onset of the LD cycle.
In addition to being self-sustaining in the absence of periodic input, endogenous clocks free-run at their intrinsic period (e.g., ~circadian) under constant conditions, have a general uniformity of period, exhibit phase resetting within a range of entrainment when exposed to a time-giver (zeitgeber), tend to be temperature compensated, have a genetic basis, and most importantly, they are used to order biological events 2.
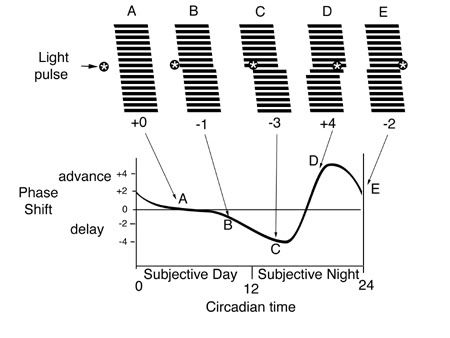
Because the endogenous oscillator seldom exactly matches the period of the exogenous pacemaker, a mechanism must be in place to daily re-adjust the endogenous period to the exogenous one. In humans, light pulses in early subjective night produce a delay in the endogenous rhythm, shifting sleepiness to later in time, whereas light pulses late in subjective night advance the rhythm to promote earlier wakefulness. The exact timing of light determines the size of the phase shift on the phase response curve, and there is a sudden reversal between phase delays and advances around mid-subjective night. At other times, light has no effect on the phase of the endogenous rhythm, producing a so-called “dead zone” in the phase response curve.
Figure 2. Illustration of the construction of a phase response curve for a nocturnal mammal. The animal is allowed to free-run (tau = 25 h) under constant darkness. Pulses of light are given at various circadian times (A-E), i.e., during the animal’s subjective day and night to test how far and in what direction (advances or delays) the rhythm shifts. The phase shift is plotted as a function of circadian time (adapted from 2).
The Suprachiasmatic Nuclei (SCN)
The so-called “master,” light-entrainable oscillator was identified in 1972 independently by two groups as small, bilateral nuclei at the base of the hypothalamic brainstem, sitting atop the optic chiasm and known as the suprachiasmatic nuclei 8,9. The first group noted that ablation of the SCN resulted in disruption of behavioral rhythms, whereas the other noted disruption of endocrine rhythms.
Although the resulting clock function of the SCN appears rather singular, individual phenotypically and functionally differentiated cells within the SCN themselves contain autonomous molecular clocks, some expressing “clock genes” rhythmically via autoregulatory transcription-translation feedback loops, often having idiosyncratic phases and free-running rhythms, and others showing light-inducible clock gene expression. Together, these 20,000 cells compose a heterogeneous population of unique time signatures in a functionally coherent web of coupled oscillators sensitive to the celestial clockwork, and photic stimuli, more generally 10. In this heterogeneous web, the miniscule master clock itself is like a minor fractal mirror on the organization of the multi-oscillatory organism as a whole.
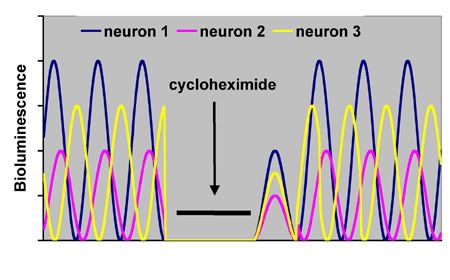
Individual SCN pacemaker cells and their relationships can be directly observed in vitro in hypothalamic slices from a transgenic animal containing a bioluminescent reporter gene that is activated by a clock gene such as Per1 maintained in a cell culture medium 11.
Figure 3. A hypothetical illustration of individual SCN neurons in vitro expressing a bioluminescent luciferase reporter for the period gene per1 (adapted from 11). Note that individual neurons show different amplitudes and phase relations prior to treatment (free-running periods were kept the same for purposes of illustration). The protein synthesis inhibitor cycloheximide is applied to the medium to prevent cycling of the molecular clock, thus preventing further oscillations in the observed rhythm. Upon cessation of cycloheximide, the individual neurons resume oscillating at reduced amplitude, but now at the same phase relationship for a cycle or two, before resuming their original phase relationships as they were prior to cycloheximide. Thus, the phase relationships between neurons are self-organizing, analogous to the self-coordinating pendulums on Huygens’ clocks.
In addition to receiving photic information directly from light transduction pathways and indirectly from sensory thalamic pathways, the SCN are deeply embedded within the motor brainstem, neurally projecting to hypothalamic behavioral, neuroendocrine, and autonomic motor zones, as well as a major intra-hypothalamic pattern generator that additionally coordinates rhythmic inputs and aperiodic, adventitious inputs to these motor outflows. Thus, the SCN are situated in the crossroads of sensitivity to the celestial clockwork and endogenous motor organization, and unexpected events, as well.
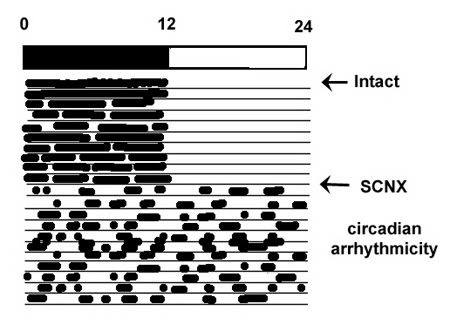
Animals bearing lesions of the SCN are unable to entrain to light:dark cycles, and rhythms in behavioral, neuroendocrine, and autonomic outflows become completely arrhythmic in the circadian domain, although some shorter ultradian rhythms may persist (Fig 4). Circadian rhythmicity can restored by SCN transplants, with the intrinsic free-running period corresponding to that of the donor animal, indicating a genetic component to the clockwork. Even when transplants are encased in a permeable membrane to prevent any potential neural grafting with host tissue, the SCN continue to restore rhythmicity, indicating a capacity for humoral, as well as neural communication.
Figure 4. An illustration of the effect of SCN lesions on a sample slave rhythm. Under a 12:12 dark:light cycle, an intact nocturnal animal, such as a rat, is active during the dark phase of the DL cycle. Almost immediately following ablation of the SCN (SCNX), the animal’s rhythms become completely arrhythmic in the circadian time domain, although shorter (ultradian) rhythms may persist.
In addition to rhythmic control over behavioral and autonomic outflows as illustrated in Fig. 1, the SCN exert control over rhythmic neuroendocrine (hormonal) outflows controlling multiple physiological processes, such as metabolic, growth, and reproductive cycles. For the purpose of illustrating the hierarchical control of the master pacemaker over various slave oscillators, the metabolic axis provides an excellent example, and will lead us to a comparison of inducible auxilliary oscillators when photic stimuli and the SCN no longer suffice for survival. Although frequently mistaken as a “stress axis,” the hypothalamo-pituitary-adrenal (HPA) axis peaks daily under unstressed conditions of simply waking up, and is rather best understood as an axis of metabolism.
Briefly, the HPA axis consists of Hypothalamic motorneurons expressing corticotropin releasing factor (CRF), which stimulates Pituitary corticotrophs to release adrenocortipcotropin hormone (ACTH), which then enters the blood stream to stimulate the tiny Adrenal glands sitting atop the kidneys to release corticosteroids into the bloodstream to effect protean effects throughout the organism. Like most endocrine systems, activation and secretion events are episodic and pulsatile, occurring in the human HPA axis as an ultradian rhythm having a period of about an hour.
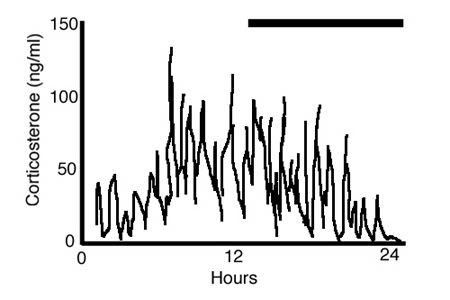
Figure 5. An illustration of corticosterone secretion in a female rat over 24 h (dark bar represents lights out). With rapid, e.g., 10-min sampling, the pattern of glucocorticoid secretion is clearly pulsatile with an ultradian period of around an hour. Super-imposed on this ultradian pulsatility is a clear circadian rhythm that peaks at around the time of the active period (the dark period for rats). Adapted from Lightman et al 12.
The SCN super-impose circadian control over pulsatile secretion in several ways. The SCN project directly to the HPA axis neurosecretory neurons of the PVN to directly active motorneurons. The SCN also indirectly to those same neurons via secondary pattern generators in structures surrounding the motor zone. Finally, the SCN projects to the autonomic nervous system to control sensitivity of the adrenal gland to upstream products. In addition, each level of the HPA axis contains its own clock genes that appear to be synchronized with the master LEO (see below).
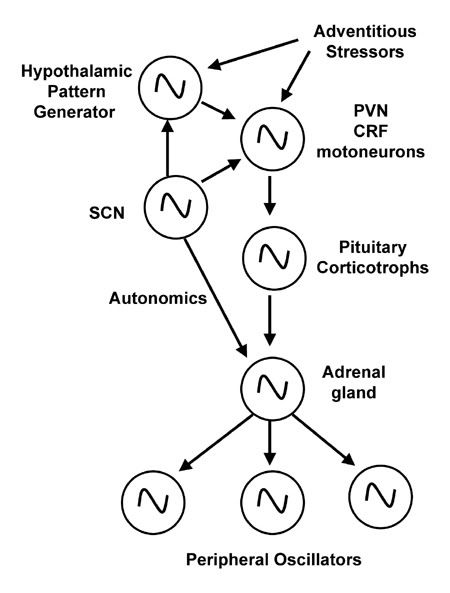
Figure 6. An illustration of the quasi-hierarchical web of oscillators and pattern generators that control HPA axis outflow. At the top of the hierarchy is the light-entrainable oscillator (SCN) which projects to the pinnacle of the HPA axis, the corticotropin releasing factor (CRF) motorneurons, as well as an intra-hypothalamic pattern generator that integrates rhythmic and adventitious inputs and in turn projects to the HPA axis. CRF neurons stimulate ACTH secretion from the pituitary corticotrophs which then enters the bloodstream to stimulate the adrenal cortex to release glucocorticoids. Glucocorticoids then act on a host of peripheral and central targets, frequently acting as an orchestrator of peripheral oscillators.
Corticosteroids released by the adrenal then act on multiple target tissues, such as the liver and brain, most of which also contain both glucocorticoid receptors (a notable exception being the SCN) and functional clock genes. Glucocorticoids acts as important synchronizing signals, as peripheral tissue oscillations often desynchronize in the absence of entrainment signals. For example, a single pulse of glucocorticoids resets rhythmic, but asynchronous cultured fibroblast cells to a common circadian phase in vitro 13,14. Likewise, a single pulse of the synthetic glucocorticoid dexamethasone re-establishes rhythmic activity of clock genes in the hepatocytes of mice bearing SCN lesions in vivo 15. In the central nervous system, glucocorticoids rapidly cross the blood brain barrier to act on diverse processes such as dopamine and CRF efflux, hippocampal theta rhythms, and clock gene expression 16,17, thus affecting multiple oscillatory processes related to motivation, mood, memory, and executive processing, thus providing internal synchronization to multiple metabolic and motivational processes 18.
Inducibile Clocks: The Food-Entrainable Oscillator (FEO)
Under ideal conditions, wherein environmental pacemakers comply with genetically predicted life-strategies, i.e., when diurnal, nocturnal, and crepuscular species can expect to eat during their active periods (day, night, and at dawn and dusk, respectively), respective LEOs produce graceful clockwork to ensure that motivational and metabolic systems “wake up” to their respective tasks of matching behavior, autonomic, and neuroendocrine outflows to life-sustaining outcomes.
But what happens when food is no longer available during an organism’s active period? Should the organism remain enslaved to the dictates of the master oscillator when critical metabolic resources become unpredicted by the master oscillator? Like Linnaeus’s clockwork flowers, some species are best preyed upon at certain times of day or season, depending upon the niche. If energy is the coin of the realm in the Kingdom of Life, then an organism would be well-suited to be able to over-ride, dominate, or at least not be completely enslaved by the master clock.
One key observation suggesting food could act as a zeitgeber was when Richter 19 showed that rats would show anticipatory wheel running in response to restricted feeding times (August Forel had previously observed similar behavior in honey bees). A second was Krieger’s observation that the HPA axis would also anticipate once-daily meals when meals were pitted against a light/dark cycle, which was thought to be the singular driver of the HPA axis 20,21. Recent decades improved considerably upon these early observations, showing that all major motor outflows, multiple brain regions and peripheral organs, and digestive processes can re-organize around feeding times particularly under conditions of food stress. Circadian food-tracking independent of light cues has been shown in species as diverse as bees, fish, marsupials, birds, and various rodents and primates 22,23.
The food-entrainable circadian system, the putative, yet still hypothetical underlying oscillator of which is provisionally referred to as the food-entrainable oscillator (FEO), holds distinct similarities and differences with the LEO (See Fig. 7). First, rather than being continuously self-sustaining, the FEO appears to be an inducible clock, also referred to as a “swing-type” or “damped” oscillator, one set in motion by specific events and damping out once induction conditions desist. The main conditions for induction appear to be food deprivation and the provision of meals achieving a certain caloric threshold, or in some cases, a mere memory of a former threshold-sized meal. Second, the FEO runs independently of the LEO. Like the LEO, it is considered a true oscillator as it can persist for at least two cycles in the absence of a food zeitgeber, distinguishing it from interval timing processes, e.g., egg-timer or hourglass mechanisms that run only one cycle to completion. The FEO is reset by food pulses, shows stable phase and period control by food, i.e., it entrains to food, shows a range of entrainment (Tau ~23-27 h) and can exhibit a free-running period (~26 h) similar to the LEO.
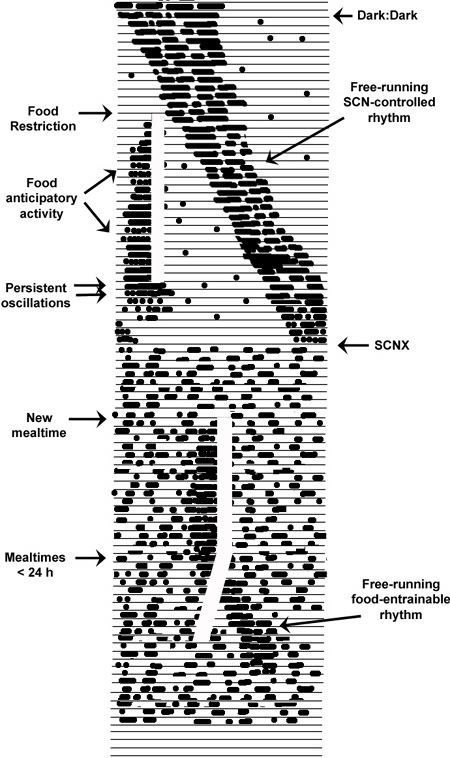
Figure 7. A hypothetical illustration of the food-entrainable oscillator’s (FEO’s) operating characteristics (each line = 24 h). Under constant conditions of DD, rhythms controlled by the LEO free-run at > 24 h. A regimen food restriction and once-daily meals begins at the white vertical bar. Fairly rapidly food anticipatory activity begins occurring in the hours prior to mealtime, independently and at virtually any phase relation to the LEO-controlled rhythms, showing both phase and period control by food. The food zeitgebers is withheld for several days to probe oscillatory capacity, and meal-related rhythms persist for at least two days in the the absence of food. When animals undergo ablation of the SCN, they become completely arrhythmic in the circadian domain. A new restricted feeding time is established and food anticipatory activity again emerges prior to meals, demonstrating complete independence from the LEO. Finally, the period of the food zeitgeber is shortened beyond the range of entrainment of the FEO and the feeding rhythm breaks away and begins free-running at its own preferred intrinsic period (See 23-28).
Although entrainment to meal times can take anywhere from 2-14 days, bigger meals and regular feedings times produce more rapid entrainment. Nevertheless, elegant experiments using non-circadian schedules of feeding have shown that single meals are sufficient to at least partially reset the clock. White and Timberlake, for example, used a 31-h schedule of meal presentation based on the logic that 31 h should be outside the range of entrainment of a circadian oscillator, while still showing the effects of single meals on the underlying clock. A second advantage of this schedule is that it advances through local time by 7 h each day, and never repeats the same local time of day for 24 days, thus avoiding any repetition of local cues. Thus, they argued, if the FEO is reset on a meal-by-meal basis, meal-related activity should ensue from the last meal taken by a circadian period rather than anticipate the next meal. This is precisely what happened. When the data were folded at a 31-h period, peak meal-related activity was observed at around 26 h after the last meal, the presumed intrinsic period of the FEO, but did not anticipate the next meal 29,30. Thus, the FEO can track meals repeated at the same time of day (within a range of entrainment), as well as constantly shifting meal times, during periods of maximum food uncertainty.
The locus of the FEO remains unknown, despite heroic efforts to localize it to a single structure in the brain or periphery, leading many to speculate that the food entrainable system is distributed across multiple oscillatory tissues that become coupled under conditions of food stress. The fact that clock gene expression in individual cultured cells can be phase shifted by glucose is consistent with this view. However, some structures appear more critical than others. For example, the DMH appears to be an excellent candidate as a major node anatomically and functionally in the generation of food-entrainable rhythms, as it is situated as the major node of a hypothalamic pattern generator controlling behavioral, neuroendocrine, and autonomic outflows.
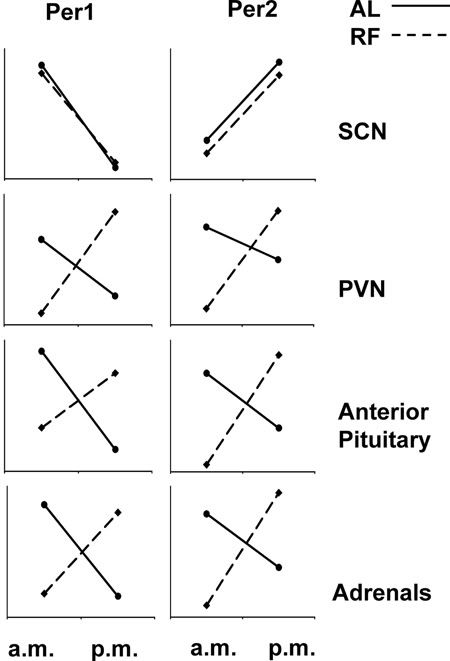
Figure 8. Illustration of the de-coupling of HPA axis clock genes from the master, light-entrainable clock (the SCN) under conditions of restricted feeding in a nocturnal mammal (rat). Under ad libitum feeding (solid line), Per1 mRNA peak in the a.m. and reach their nadir at night, whereas Per2 mRNA show the opposite pattern in the SCN and at all levels of the HPA axis. When food is restricted to the morning (dashed line), the expression of clock genes is unaffected in the LEO, whereas expression of both gene products reverses throughout the axis. Thus, the metabolic axis decouples from the light-entrainable circadian in order to meet the metabolic and motivational demands of procuring restricted meals (data adapted from 31).
Circannual Rhythms: Those dying generations.
…The young
In one another’s arms, birds in the trees
– Those dying generations – at their song,
The salmon-falls, the mackerel-crowded seas,
Fish, flesh, or fowl, commend all summer long
Whatever is begotten, born, and dies.
Caught in that sensual music…–W.B. Yeats
Thus far, we have focused on adaptive rhythmicity in the range of the solar day, but changes in the celestial clockwork across seasons can be starker than night and day, especially in more polar climes. Thus, circannual rhythms provide a mechanism for organisms to anticipate the rigors of seasons and optimize the timing of reproduction. Numerous species, from butterflies to fish to birds to whales to mountain sheep, exploit seasonal changes in global energy distribution for metabolic and reproductive purposes, resulting in great seasonal migrations and dispersals and circannual reproductive regimens of mating and territorial defense corresponding to changes in day length, temperature, and food availability. The major ultimate factor in reproductive succes is energy availability, whereas the major proximate factor is photoperiod, both from day-to-day and season-to-season 32,33.
By analogy to dawn and dusk light transitions that signal circadian physiology, light transitions occurring at spring and fall signal circannual physiology. In many species, day length appears to be a critical cue in determining changes in reproduction, e.g., gonadal actvity, pregnancy, lactation, metabolic variables such as fat deposition, molt cycles, e.g., pelage, antlers, feathers, and migratory restlessness. Especially for species living in temperate regions, diminishing day length often signals a regression of reproductive organs, increased fat deposition, and an urge to migrate to more equatorial climates, whereas increasing day length signals the recrudescence of reproductive organs, increased energy expenditure, and an urge to return to, defend, sing and display about, and mate upon and engage in parental behavior in specific natal territories. Even minor e.g., 17 minutes, changes in day length can affect reproductive status in equatorial species .
Circannual rhythms possess critical features of true endogenous clocks, including showing persistence under constant conditions (albeit sometimes weakly), free-running with a period slightly different from the annual zeitgeber (usually shorter than one year), and entrainment (phase and period control) by the zeitgeber (day length). Each characteristic may vary with species, latitude, and life history strategies. For example, the migratory restlessness (a.k.a. “Zugunruhe”) of long-distance migratory birds persists for many years without damping or loss of precision, whereas a related, but weakly migrating species may damp out after several cycles under laboratory conditions 34.
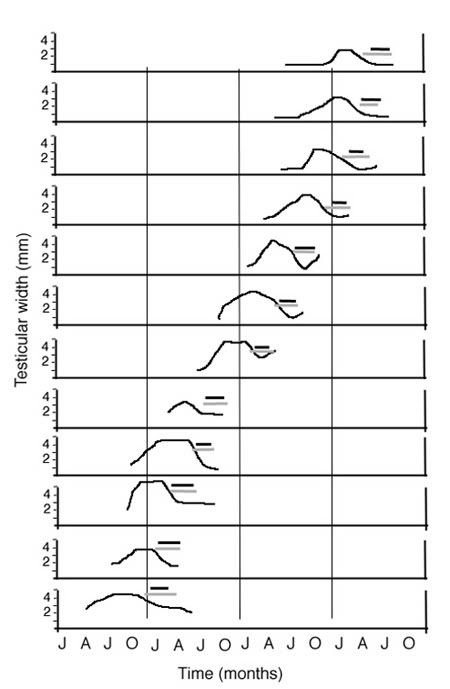
Figure 9. An illustration of circannual rhythms in testicular size (y-axis) and molting in a single male African stonechat under a 12:12 LD cycle. Black bars indicate flight feather molting, whereas gray bars indicate body feather molting. All three variables appeared to free-run with respect to yearly timing across a 12-year measurement period. In this case, the free-running circannual period was approximately 9 months, thus advancing 3 months per year.
Multiple hypotheses concerning the proximate mechanisms of circannual clocks exist. According to one account, animals may merely count days (circadian cycles) to derive a specific calendar time. However, there is no evidence that animals having shorter free-running circadian cycles experience shorter calendar times. A second suggests that animals use a “sustained hourglass” process, in which a life-cycle stage in a sequence of life-cycle stages sets in motion the next stage, as a circular sequence of finite state machines. This “meta-clock” version fails to account for species in which some stages, e.g., gonadal cycling, but not all, e.g., molt cycles, persist under constant conditions 33. For the scope of this essay, we will concern ourselves with a third promising alternative, one that applies across many, but certainly not all species exhibiting circannual rhythms.
The Master Circadian Clock and Seasonal Melatonin Rhythms.
The average suburbanite or city-dweller may experience a “sleep hangover” after waking in a setting far from city lights, e.g., a mountain cabin, where night-times are relatively pitch black. The first draughts of such sleep can seem an unexpectedly deep, saturating and slaking refreshment, if somewhat disorienting upon waking. One correctly surmises that the unusually inky night-time is causally related alterations in sleep onset, consolidation, and a relative increase in brain electrical activity associated with rapid eye movements 35.
Melatonin is secreted nightly based on signals obtained from the master circadian oscillator via its projections to sympathetic neurons that stimulate the pineal gland to secrete melatonin. Melatonin is also exquisitely sensitive to (inhibited by) even the smallest number of photons impinging upon photoreceptors that project to the pineal from the retino-hypothtalamic tract. Thus, melatonin secretion is roughly proportional to night length and overall ambient light levels. As latitude becomes less equatorial and more polar, day length becomes more variable with changes in seasons, thus directly affecting the duration and amplitude of the nightly melatonin pulse, thus providing an exogenously derived seasonal signal 36.
Calendar cells.
The melatonin signal may be integrated by calendar cells in the pituitary, specifically in the pars tuberalis (PT) region that is rich in melatonin receptors (mt1). PT cells appear to register the duration of the melatonin signal to then control the secretion of prolactin from the lactotrophs in the pars distalis via prolactin releasing factors (tuberalins) that are up-regulated in long days and down-regulated under short days. The duration decoding of the melatonin signal appears to result from progressive sensitization and desensitization of intracellular signaling (hazlereigg, 1993; von Gall, 2002). In the a seasonal rodent such as the Siberian hamster the weaker melatonin signal during long days allows a higher amplitude of morning clock gene (period) expression in the calendar cells, whereas the longer melatonin signal during short days results in the suppression of clock gene amplitude. Sheep, on the other hand, appear to interpret the melatonin signal based on the phase relationship between two clock genes. Under long days, there is a long interval between the morning disinhibition of period gene and evening activation of cryptochrome gene expression, whereas in short days that interval is reduced, thus signaling summer and winter phenotypes, respectively, downstream 36.
However, it is unlikely that the duration of melatonin signaling alone accounts for the circannual timer, as animals kept in constant conditions, and hence under constant melatonin signaling, and even animals having constant-release melatonin clamps, often continue to express circannual cycles in physiology. The mechanism for any underlying long-term counters or oscillators aside from melatonin signaling have yet to be identified. Further, there are likely central sites controlling other aspects of seasonal physiology, as lesions in the hypothalamus can prevent photoperiod-induced changes in, say, the gonadotropin axis 37.
Conclusions.
Someone said, “Time is what prevents everything from happening at once.” Said another way, time is what allows organized complexity, dynamism, optimization and adaptation. The major endogenous oscillators discussed here, the light-entrainable, food-entrainable, and circannual clocks, are not dependent upon or even necessarily associated with time perception, that is, the phenomenal feeling of the passage of time, but are rather more fundamental, subliminal, automatic processes constantly at work to maintain massive cascades of physiological order, automatically tuning the body to the vibrations of the local cosmos from hour to hour, day to day and season to season.
References
1. Linnaeus C. Philosophia Botanica. Stockholm: Godofr Kiesewetter; 1751.
2. Moore-Ede MC, Sulzman FM, Fuller CA. The clocks that time us : physiology of the circadian timing system. Cambridge, Mass.: Harvard University Press; 1982. xii, 448 p.
3. Duhamel DuMonceau HL. La Physique de Arbres. Paris: H.L. Guerin and L.F. Delatour; 1759.
4. Zinn JG. On the sleep of plants. Hamburgisches Magazin 1759;22:40-50.
5. Aschoff J. Circadian Rhythms in Man. Science 1965;148:1427-32.
6. Bennett M, Schatz MF, Rockwood H, Wiesenfeld K. Huygens’s Clocks. Proceedings: Mathematical, Physical and Engineering Sciences;458(2019):563-579.
7. Wever RA. The Circadian System of Man: Results of Experiments under Temporal Isolation. New York: Srpinger Verlag; 1979.
8. Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Procedings of the National Academy of Sciences (USA) 1972;69:1583-1586.
9. Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Research 1972;42:201-206.
10. Antle MC, Silver R. Orchestrating time: Arrangements of the brain circadian clock. Trends Neurosci 2005;28(3):145-51.
11. Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science 2003;302(5649):1408-12.
12. Lightman S, Wiles CC, Atkinson HC, Henley DE, Russell GM, Leendertz JA, McKenna MA, Spiga F, Wood SA, Conway-Campbell BL. The significance of glucocorticoid pulsatility. Eur J Pharmacol 2008;583:255-62.
13. Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 2000;289(29 Sept 2000):2344-2347.
14. Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. Circadian gene expression in individual fibroblasts: cell-autnomous and self-sustained oscillators pass time to daughter cells. Cell 2004;119(5):693-705.
15. Reddy AB, Maywood ES, Karp NA, King VM, Inoue Y, Gonzalez FJ, Lilley KS, Kyriacou C, Hastings MH. Glucocorticoid signaling synchronizes the liver circadian transcriptome. Hepatology 2007;45(6):1478-88.
16. Segall LA, Perrin JS, Robinson B, Rodaros D, Amir S. Exogenous corticosterone restores rhythmic expression of the clock gene, per2, in the central extended amygdala in adrenalectomized rats. Program No. 60.3. 2005 Abstract Viewer/Itinerary Planner. Washington DC: Soc Neurosci, 2005. Online. 2005.
17. Segall LA, Perrin JS, Walker CD, Stewart J, Amir S. Glucocorticoid rhythms control the rhythm of expression of the clock protein, Period2, in oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in rats. Neuroscience 2006;140(3):753-7.
18. Pecoraro N, Dallman MF, Warne JP, Ginsberg AB, Laugero KD, la Fleur SE, Houshyar H, Gomez F, Bhargava A, Akana SF. From Malthus to motive: How the HPA axis engineers the phenotype, yoking needs to wants. Prog Neurobiol 2006;79(5-6):247-340.
19. Richter CP. Animal behavior and internal drives. Quarterly Review of Biology 1927;II No 3:307-343.
20. Krieger DT. Food and water restriction shifts corticosterone, temperature, activity and brain amine periodicity. Endocrinology 1974;95:1195-1201.
21. Krieger DT, Hauser H, Krey LC. Suprachiasmatic nuclear lesions do not abolish food-shifted circadian adrenal and temperature rhythmicity. Science 1977;197:398-399.
22. Mistlberger R. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev 1994;18(2):171-95.
23. Stephan FK. Food-entrainable oscillators in mammals. In: Takahashi J, Turek F, Moore R, editors. Circadian Clocks. Volume 12, Handbook of Behavioral Neurobiology. New York: Kluwer Academic/Plenum; 2001. p 223-246.
24. Stephan FK. Resetting of a circadian clock by food pulses. Physiol Behav 1992;52(5):997-1008.
25. Stephan FK. Resetting of a feeding-entrainable circadian clock in the rat. Physiol Behav 1992;52(5):985-95.
26. Stephan FK. Calories affect zeitgeber properties of the feeding entrained circadian oscillator. Physiology & Behavior 1997;62(5):995-1002.
27. Stephan FK. The “other” circadian system: food as a Zeitgeber. J Biol Rhythms 2002;17(4):284-92.
28. Mistlberger RE. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neuroscience & Biobehavioral Reviews 1994;18:171-195.
29. White W, Schwartz GJ, Moran TH. Meal-synchronized CEA in rats: effects of meal size, intragastric feeding, and subdiaphragmatic vagotomy. American Journal of Physiology-Regulatory Integrative and Comparative Physiology 1999;45(5):R1276-R1288.
30. White W, Timberlake W. Meal-engendered circadian-ensuing activity in rats. Physiology & Behavior 1999;65(4-5):625-642.
31. Girotti M, Weinberg M, Spencer R. Diurnal expression of functional and clock-related genes in the rat hypothalamus-pituitary-adrenal axis: System-wide shifts in response to restricted feeding. Am J Physiol Endocrinol Metab 2009.
32. Gwinner E. Circannual rhythms in birds. Curr Opin Neurobiol 2003;13(6):770-8.
33. Wikelski M, Martin LB, Scheurlein A, Robinson MT, Helm B, Hau M, Gwinner E. Avian circannual clocks: Adaptive significance and possible involvement of energy turnover in their proximate control. Philos Trans R Soc Lond B Biol Sci 2008;363(1490):411-23.
34. Gwinner E. Circadian and circannual programmes in avian migration. J Exp Biol 1996;199:39-48.
35. Dijk D, Cajochen C. Melatonin and the circadian regulation of sleep initiation, cosolidation, structure, and the sleep EEG. J Biol Rhythms 1997;12(6):627-35.
36. Lincoln GA, Andersson H, Loudon A. Clock genes in calendar cells as the basis of annual timekeeping in mammals–a unifying hypothesis. J Endocr 2003;179:1-13.
37. Maywood ES, Hastings MH. Lesions of the indomelatonin-binding sites of the mediobasal hypothalamus spare the lactotropic but block the gonadotropic response of male Syrian hamsters to short photoperiod and to melatonin. Endo 1995;136(1):144-53.

4 comments
Skip to comment form
Author
it’s some kind of change, some kind of spinning away, with every single line moving further out in time.
Can’t find the Eno music vid “Spinning Away,” dammit, and you deserved it if you made it even part way through this unbearably tedious post on what is truly an almost , musical, magical topic.
…FEOs, SNCs, Oscillations or variable circadian rhythms. but I do love the order of the universe beyond imagination, beyond extinction. You bet!
And thus, more from “The Idea of Order at Key West”
~~~~~~~pause
Just an excerpt. Compound F quoted the first stanza at the top of his essay. My quote is in the middle. I urge everyone to find this poem and read it. It is wonderous, and it fits CF’s ideas of how we-the-universe-create-created-continue-generate.