The germ of this piece came from an undertaking that I am considering. That undertaking is to write a post for every chemical element. The recent successes of my more technical pieces have made me decide to concentrate more on the harder part of science rather than less technical material.
The problem with that is that it would take over two years to cover all of the elements, and in reality even longer because there are topics out there that will surely be more topical. I am not sure that this is feasible. Maybe I could look at families, but then that gets way too general. Any thoughts on how to approach (or even if I should) this huge array of subjects would be appreciated.
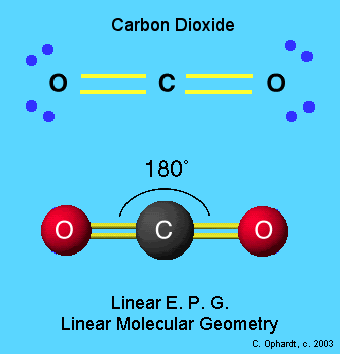
In any event, I would start with hydrogen and work my way to heavier elements. One of the first things that came to mind was the isotope effect, because hydrogen has the largest isotope effect of any element. Please stay with us!